Oncomine™ Lung cfDNA Assay
Introduction
Cancer researchers know every tumor is different. While sampling tissue remains standard practice, there are likely several advantages to liquid biopsy samples: a) they enable serial analysis of multiple samples from the same subject; b) they potentially enable better analysis of tumor heterogeneity; c) they can be collected in cases where tissue samples cannot be collected, as is frequently the case for lung cancer samples; and d) they are less invasive and less expensive to obtain than tissue samples. Of interest is blood borne cfDNA that originates from a tumor and is called circulating tumor DNA (ctDNA). ctDNA is rare and represents a low percentage of mutant DNA (0.1–1.0%) in typical samples.
To unlock the potential in liquid biopsy samples, translational and clinical researchers need an NGS assay that enables multi-biomarker analysis at low mutation frequencies to detect primary tumor drivers and resistance mutations. The Oncomine Lung cfDNA Assay introduces a workflow for the isolation of cfDNA from a single blood sample followed by variant analysis using the Ion S5™ XL System, and a trained variant caller plug-in in Torrent Suite™ Software.
Methods
To isolate cfDNA from a 10 mL blood sample, the sample was centrifuged to isolate the plasma fraction. Using the Applied Biosystems™ MagMAX™ Cell-Free DNA Isolation Kit manual protocol, cfDNA was recovered from the plasma. From the starting sample, 5–20 ng cfDNA was obtained from healthy tissue samples and 5–100 ng cfDNA was obtained from late-stage lung cancer samples. Purified cfDNA from plasma was amplified using the Oncomine Lung cfDNA Assay following standard library construction as outlined in the user guide.
Analysis
Sequencing data were analyzed using Torrent Suite Software version 5.2 or higher cfDNA variant caller plugin with parameters optimized for the Oncology-Liquid Biopsy application.
Results
The Oncomine Lung cfDNA Assay targets key genes in NSCLC: ALK, BRAF, EGFR, ERBB2, KRAS, MAP2K1, MET, NRAS, PIK3CA, ROS1, and TP53. Using matched tissue and blood from late-stage NSCLC samples, a high correlation was observed between the variants called in formalin-fixed, paraffin-embedded (FFPE) research samples and those called in cfDNA from plasma (Table 1). There is also a higher allelic fraction in the FFPE tumor samples compared to that measured in plasma when the variant is derived from cancer cells. As well, germline variants in MET-T1010I are seen at close to 50% in both FFPE and plasma in two cases.

Table 1. Mutation correlation between FFPE samples and cfDNA1.
With mean sensitivity at 90%, mean specificity at 98%, observed performance of the Oncomine Lung cfDNA Assay at 0.1% LOD enables amplification of more difficult research samples (Table 2). In addition to these measurements of expected variants in control samples, the actual cfDNA samples tested had less than one false positive in more than 8 libraries, indicating very high specificity for calling low-frequency variants in cfDNA. To verify this performance, Oncomine Lung cfDNA libraries were generated with engineered controls from Horizon Discovery™ as input. Data in table 3 show the variant frequencies called by the variant caller analysis plug-in for the 8 somatic variants engineered into the Horizon Discovery™ Multiplex cfDNA Reference Standard Set when analyzed using the Oncomine Lung cfDNA Assay.
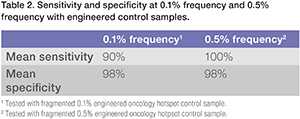
Table 2. Sensitivity and specificty at o.1% frequency and 0.5% frequency with engineered control samples.

Table 3. Variants called from Multiplex cfDNA Reference Standard Set
Conclusion
The Oncomine Lung cfDNA Assay contains 35 amplicons covering >150 SNV hotspots across 11 genes. Coupled with the Ion S5 System, this research assay enables efficient multi-biomarker analysis of cfDNA in blood. With flexible limits of detection as low as 0.1% with high specificity and sensitivity, clinical cancer researchers may detect ctDNA sooner to better research resistance mutations as they emerge.






