Breaking Through the Barriers to Lab Innovation

Complete the form below to unlock access to ALL audio articles.
Introduction
Innovation is a hot topic today and just about every type of laboratory is scrambling to figure out what it means for them. Lab Managers are expected to design profitable new products that enable the research organization to stay competitive in today’s marketplace. This means change. Process change. Systems change. Informatics technologies change. As a result, systemic change is occurring at all levels of the organization, driving the implementation of integrated lab solutions that unlock disparate, disconnected lab data silos and harmonize the IT infrastructure. Getting greater control of lab data is part of this and one of the most critical components of future success and corporate sustainability. As a result, some of the greatest change is taking place in Informatics in laboratories around the world.
Two of the most significant barriers to innovation are outdated informatics tools and inefficient workflows. Moving from paper-based manual methodologies to digital solutions can breathe new life into researcher productivity while enabling forward-looking companies to better compete and excel in today’s rapidly changing business environment.
This article examines the drivers behind the move for greater innovation, challenges, current trends in laboratory informatics, and the tools and techniques that can be used to break through barriers to lab innovation. Several leading informatics vendors provide their views.
Selected Vendors

Laboratories worldwide seeking a single, integrated informatics platform can now standardize on one comprehensive laboratory information management system (LIMS). Thermo Fisher’s integrated informatics solution now comprises method execution, data visualization and laboratory management, and seamlessly integrates with all popular enterprise-level software packages.
“Thermo Scientific SampleManager is a fully integrated laboratory platform encompassing laboratory information management (LIMS), scientific data management (SDMS) and lab execution (LES).”
Trish Meek, Director Strategy, Informatics, Thermo Fisher Scientific
More Information
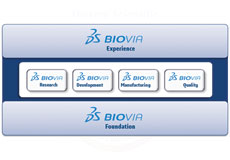
BIOVIA Unified Lab Management allows for streamlined and more efficient lab workflows and a fully integrated and automated easy-to-deploy process. Based on the BIOVIA Foundation it works as an integration hub for BIOVIA applications as well as all major 3rd party systems and instruments allowing for seamless data transfer.
“BIOVIA Unified Lab Management is part of our unique end-to-end Product Lifecycle support for science-based organizations to improve innovation, quality, compliance, and efficiency.”
Dr. Daniela Jansen, Senior Solution Marketing Manager
More Information
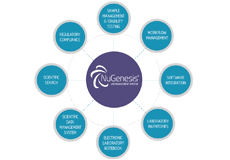
Waters® NuGenesis® Lab Management System uniquely combines data, workflow and sample management capabilities to support the entire product lifecycle from discovery through manufacturing. This user-centric platform encompasses NuGenesis SDMS, compliance-ready data repository, NuGenesis ELN, a flexible analytical electronic laboratory notebook, and NuGenesis Sample Management.
“The NuGenesis LMS readily adapts to existing informatics environments, smoothly linking data from the lab to the business operations of a company, so science-driven organizations can see more, know more and do more.”
Garrett Mullen, Senior Product Marketing Manager, Laboratory Management Informatics, Waters
More Information
The Impact of Corporate Wide Initiatives
There are a number of sweeping changes occurring throughout the corporate world that are turning the spotlight on research laboratories, examining everything from workflows to documentation. These changes are driven by corporate initiatives to increase profits, reduce costs, develop new products and drive operational efficiencies throughout the enterprise. These are not new goals, but the methodologies for achieving these goals have changed significantly thanks to the rapid changes in technology. Now, there is a greater focus on how technology can drive innovation throughout the enterprise.
In fact, almost every leading multinational organization nowadays touts innovation as an underlying theme for how they conduct business and develop the next generation products. To be truly innovative however, businesses of all types must embrace innovation at every level of the enterprise – not just in the products under development, but also how those products are being developed.
“Organizations nowadays cannot afford to not look into innovation,” emphasizes Dr. Daniela Jansen, Senior Solution Marketing Manager at BIOVIA. “Now, they are questioning how product quality is being supported by innovation throughout the end-to-end product lifecycle. The time is past when researchers looked to a single functionality to make a difference. Now, all software needs to drive innovation, to drive costs down and to drive efficiency.”
Garrett Mullen, Senior Product Marketing Manager at Waters Corporation, offers another perspective. “We drive innovation by addressing the challenges. Sometimes it is specific to the market, such as petrochemical or pharmaceutical, sometimes it is specific to the task, such as sample registration for the QA/QC department. All markets are suffering from similar challenges, whether it is products coming off patent or waning market share. So there is a big focus on what they can do about it, from controlling costs to simplifying processes.”
Operational excellence plays a significant role in corporate initiatives for innovation, and this is where the initiatives drill down into the research laboratories. According to Trish Meek, Director of Product Strategy for the Informatics business at Thermo Fisher Scientific, “Executives are looking more closely at the lab as part of a more holistic view of operational efficiencies across the entire organization. There’s a larger expectation than ever before that there is hidden value in the lab, and that can be found in optimizing efficiencies and more fully integrating processes across the lab and throughout the rest of the manufacturing or production process. Executive metrics now include the lab as they analyze data from all aspects of their operations in order to improve their processes, improve the quality of their products and drive profitability. Executives are now mining and reviewing data to determine how to make operations better from a holistic perspective, and that is causing the spotlight to be on the lab more than it ever was.”
A key aspect of operational excellence is that it goes hand in hand with product quality. Not only is there a need to expedite innovation to deliver new products, those new products need to be high quality and to comply with changing environmental regulations and consumer expectations. As a result, research organizations are reviewing their Informatics infrastructure and streamlining laboratory operations.
Further, the technology that supports lab Informatics has been evolving rapidly, delivering new functionality that is changing the way research can be performed. This points to the heart of the matter: current technology is enabling new workflows (such as digital collaboration) while delivering greater access to research and also enabling better examination of the research (such as through the ‘Cloud’). This paradigm shift is happening at many levels, from how research is performed to how the data is shared, with technology at the center of the shift.
Barriers to Innovation: The Migration from Paper to Digital
Legacy paper-based activities in the lab are perhaps one of the greatest barriers to innovation. Data captured in paper lab notebooks is typically difficult to find, read or share. Written observations are often transcribed incorrectly. Tests and experiments are repeated because prior data is lost or inaccessible. Even though many lab activities are conducted electronically, certain steps are often still conducted on paper. Such repetitious manual activities are one of the greatest impediments to productivity. These workflow gaps are slowly being replaced with seamless digital activities.
One of the most interesting aspects of the drive for innovation is the ability to take advantage of the technology tools now available, which deliver a significant new range of functionality to users. Electronic Lab Notebooks (ELNs), for instance, can now be connected in the Cloud so that scientists anywhere can collaborate and share research data. This is important because not only is the transition to ELN’s happening on a local level, it is part of a larger global movement toward distributed research as a result of changes in how research organizations are now managing their operations. Large multinationals with research centers distributed around the globe are enabling their scientists to collaborate easily and efficiently with ELN’s as part of their effort to streamline operations.
 “It is still surprising to see paper in the lab,” states Meek. “It’s in many cases a cultural issue – a comfort level – which makes it hard to move away from paper, and it's a system everyone knows. Despite its flaws, paper is infinitely flexible, but in general it is terribly inefficient with regards to big data and computational power. Now, the need to look at all the data, and have all the data available is far more important, meaning that the move away from paper or manual data management is now more important than ever.”
“It is still surprising to see paper in the lab,” states Meek. “It’s in many cases a cultural issue – a comfort level – which makes it hard to move away from paper, and it's a system everyone knows. Despite its flaws, paper is infinitely flexible, but in general it is terribly inefficient with regards to big data and computational power. Now, the need to look at all the data, and have all the data available is far more important, meaning that the move away from paper or manual data management is now more important than ever.”
“It continues to be about paper in many labs,” Jansen confirms. “But you need to look at the entire chain of cause and effect and the role that paper plays. Now, it’s about what drives the entire organization, not localized practices. This means that there’s a focus on reducing the time spent on documentation and removing barriers. There’s a focus on getting quality designed into the process, getting greater efficiency, and connecting the disparate silos of data the impede innovation. One way to do this is to use an open science-aware framework like the BIOVIA Foundation to integrate processes and applications from different providers. And virtual experiments that enable scientists to identify potential new products earlier in the process can significantly save time and money.“
Cost savings are one of the key reasons organizations make the transition from paper to digital practices. “We’ve found that processes went from hours to minutes when you eliminate the numerous manual review processes and transcriptions and replace them with electronic processes,” explains Mullen. “For example, in the past one central analytical lab at a company might have performed all LC [liquid chromatography] testing. Users submitted samples via email and the samples were boxed, tests requested, samples were received and registered at the central lab, etc. Very labor intensive. A digital solution changes all that. Now the new NuGenesis web interface enables the user to register the sample, enter the samples, specify the tests digitally, and thus reduce transcription errors and expedite the process. An automatic acknowledgement that the samples are approved is sent and the testing processes start. This eliminates the manual tasks associated with checking that everything is accurate. The time and cost savings are enormous.”
 Other factors are influencing the migration from paper to digital lab processes, including the recession and the heightened merger and acquisition activity. Many organizations have downsized, are running leaner, and employ fewer researchers. Yet the productivity demands remain as high as when there was more staff. Thus, there’s an increased need to ensure that researcher activities are more efficient. Manual workflows are out of sync in the digital environment.
Other factors are influencing the migration from paper to digital lab processes, including the recession and the heightened merger and acquisition activity. Many organizations have downsized, are running leaner, and employ fewer researchers. Yet the productivity demands remain as high as when there was more staff. Thus, there’s an increased need to ensure that researcher activities are more efficient. Manual workflows are out of sync in the digital environment.
Adopting Next Generation Technology
While there are numerous paper-based workflows in research labs worldwide, the vast majority of these labs have adopted some level of technology, including informatics software solutions. What began with instrument-specific software solutions, such as Thermo Scientific ChromeleonTM chromatography data system (CDS), has expanded to numerous application-specific and task-specific systems as computers have become an integral part of the lab work environment. Laboratory Information Management Systems (LIMS) have been commercially available since the early 1980’s. The increase in demand for fast turnaround and greater volumes of sample testing and analysis drove the growth in these solutions. NuGenesis® introduced the first Scientific Data Management System (SDMS) to help capture, catalog and archive lab data better in the 1990’s. ELN’s were one of the last lab systems to become a ubiquitous tool mainly because of the challenge of managing unstructured data versus structured data, but technology has overcome this issue too.
The increase in computing power accelerated the Informatics vendors’ ability to deliver faster, better, more comprehensive software tools. In parallel, the adoption of sophisticated technology by consumers created expectations for similar capabilities in the workplace, driving the demand for hardware such as tablets and other handheld devices as access tools for ELNs, LIMS and other lab software.
Yet while these different lab data and sample management systems have provided significant benefits to the lab, they started as separate systems and thus created separate data repositories that require an interface or middleware to enable data to be shared. But that challenge too is fast disappearing as new technology and new pathways to innovation arise.
“One of the things that Thermo Fisher Scientific is focused on is delivering integrated informatics,” states Meek. “Traditionally, LIMS delivered specific functionality for R&D or manufacturing labs, but didn't cover the entire laboratory process. Our customers today want an integrated solution that covers the complete lab workflow. So, we built an Integrated Informatics platform to combine many of these together so that they’re no longer separate silos with different data in different systems. Now, lab data management, method execution and scientific data management is done within the SampleManagerTM solution making its much more than just a LIMS. All of the functionality for scientific method and data management is now part of the same solution.” SampleManager has continued to evolve to offer greater functionality for our customers, so that now it has become the enabler for our customers to better manage their lab, and save their companies time and valuable financial resources formerly necessary to purchase, implement and support multiple software systems. Our goal is to continue to build upon the SampleManager platform so we can offer the greatest degree of functionality to our customers.”
“What is happening is that LIMS are now being supplemented with ELN and LES toolsets. Everyone is moving towards a center space, where LIMS become ELNs, etc.,” explains Mullen. Waters recently introduced the NuGenesis® Lab Management System (LMS) as an alternative to LIMS. Based on the NuGenesis SDMS, the LMS offers significantly more functionality that can be switched on as components are needed for various workflow and sample management tasks.
Mullen continues, “The NuGenesis LMS can create the testing protocol procedure to ensure that the tests are done correctly. It can specify the values and results, the upper and lower limits, etc., then pull the test values back into the worksheet. Results are instantly flagged as in or out of specification. If reagents are expired or an instrument needs calibration, these are flagged automatically. The result is much faster transaction times than traditional paper-based processes.”
 “For BIOVIA, when we talk about the benefits of our solutions, we’re talking about workflow efficiencies, cost savings, compliance and brand reputation,” states Jansen. “As a vendor, we support organizations by driving innovation, by strengthening the R&D pipeline while ensuring quality in their processes and outcomes. Now that BIOVIA is part of Dassault Systèmes,” Jansen continues, “we’re engaging in much larger conversations because we can now support the entire lab to plant process expanding our solutions to the 3D Experience platform. From ELNs to LIMS to virtual molecular modeling with our Discovery or Materials StudioTM solution, BIOVIA offers an integrated, unified experience that is transforming how our customers are improving product quality, collaborating across sites, reducing cycle times and reducing costs. The bottom line is the ability to rapidly, easily and accurately transfer and utilize knowledge.”
“For BIOVIA, when we talk about the benefits of our solutions, we’re talking about workflow efficiencies, cost savings, compliance and brand reputation,” states Jansen. “As a vendor, we support organizations by driving innovation, by strengthening the R&D pipeline while ensuring quality in their processes and outcomes. Now that BIOVIA is part of Dassault Systèmes,” Jansen continues, “we’re engaging in much larger conversations because we can now support the entire lab to plant process expanding our solutions to the 3D Experience platform. From ELNs to LIMS to virtual molecular modeling with our Discovery or Materials StudioTM solution, BIOVIA offers an integrated, unified experience that is transforming how our customers are improving product quality, collaborating across sites, reducing cycle times and reducing costs. The bottom line is the ability to rapidly, easily and accurately transfer and utilize knowledge.”
Each of these vendors offers a different path to a similar end, with solutions that deliver greater access to not just legacy data but also the astounding volumes of data being created in labs worldwide. The ability to turn that data into knowledge that is accessible, accurate and reusable is necessary to fuel the new product demands both inside and outside the enterprise. Next generation technology is being developed and implemented with increasing rapidity to address these market requirements.
Conclusions
Corporate demand for innovation at every level of the enterprise is helping to drive laboratory innovation, from the tools adopted to perform research to the processes used to manage that research and all the associated data, samples, reagents, tests and more.
Operational excellence has risen to the top of corporate agendas, driven in part by the availability of technology that can support a global approach to better manage the entire product lifecycle, from initial research to final product. Now, informatics solutions exist that can support every stage of the process whether the organization engages in pharmaceutical research and needs to identify promising candidates early in the process, or whether the organization develops consumer product goods that have a short product lifecycle and thus require a constant stream of new products to maintain market share.
Information integration is playing a major role in breaking through the barriers to lab innovation. As a result, there is a significant transformation underway in the informatics tools to integrate the solutions so that data is no longer inaccessible in single purpose system. For some time there have been LIMS with ELN capabilities, CDS with LIMS functions, ELNs with sample management attributes, and more. Now, the need to exchange and move data quickly and easily from one user to another has driven the availability of integrated collaborative environments that can share laboratory data cross-team, cross-location and cross organizations.
At the core of these changes is the need to more rapidly address the larger business challenges in the lab through more efficient, more market-oriented new product development. And that’s the bottom line: informatics technology can be used as an enabling tool to solve both business challenges and lab challenges. Informatics vendors all approach the market requirements differently, depending on their own corporate culture, but all strive to enable their customers to innovate.
Author: Helen Gillespie, Informatics Editor, Technology Networks

