A Better Class of Cancer Drugs

Complete the form below to unlock access to ALL audio articles.
A class of therapeutic drugs known as protein kinase inhibitors has in the past decade become a powerful weapon in the fight against various life-threatening diseases, including certain types of leukemia, lung cancer, kidney cancer and squamous cell cancer of the head and neck. One problem with these drugs, however, is that they often inhibit many different targets, which can lead to side effects and complications in therapeutic use. A recent study by San Diego State University chemist Jeffrey Gustafson has identified a new technique for improving the selectivity of these drugs and possibly decreasing unwanted side effects in the future.
Why are protein kinase–inhibiting drugs so unpredictable? The answer lies in their molecular makeup.
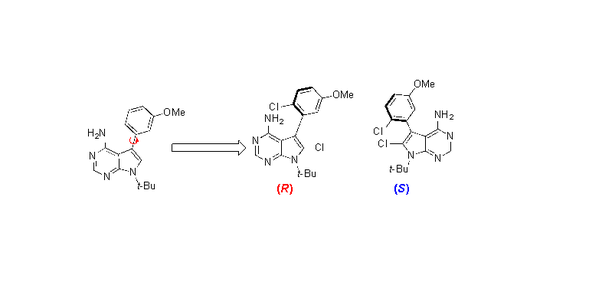
Many of these drug candidates possess examples of a phenomenon known as atropisomerism. To understand what this is, it’s helpful to understand a bit of the chemistry at work. Molecules can come in different forms that have exactly the same chemical formula and even the same bonds, just arranged differently. The different arrangements are mirror images of each other, with a left-handed and a right-handed arrangement. The molecules’ “handedness” is referred to as chirality. Atropisomerism is a form of chirality that arises when the spatial arrangement has a rotatable bond called an axis of chirality. Picture two non-identical paper snowflakes tethered together by a rigid stick.
Some axes of chirality are rigid, while others can freely spin about their axis. In the latter case, this means that at any given time, you could have one of two different “versions” of the same molecule.
Watershed treatment
As the name suggests, kinase inhibitors interrupt the function of kinases—a particular type of enzyme—and effectively shut down the activity of proteins that contribute to cancer.
“Kinase inhibition has been a watershed for cancer treatment,” said Gustafson, who attended SDSU as an undergraduate before earning his Ph.D. in organic chemistry from Yale University, then working there as a National Institutes of Health poctdoctoral fellow in chemical biology.
“However, it’s really hard to inhibit a single kinase,” he explained. “The majority of compounds identified inhibit not just one but many kinases, and that can lead to a number of side effects.”
Many kinase inhibitors possess axes of chirality that are freely spinning. The problem is that because you can’t control which “arrangement” of the molecule is present at a given time, the unwanted version could have unintended consequences.
In practice, this means that when medicinal chemists discover a promising kinase inhibitor that exists as two interchanging arrangements, they actually have two different inhibitors. Each one can have quite different biological effects, and it’s difficult to know which version of the molecule actually targets the right protein.
“I think this has really been under-recognized in the field,” Gustafson said. “The field needs strategies to weed out these side effects.”
Applying the brakes
So that’s what Gustafson did in a recently published study. He and his colleagues synthesized atropisomeric compounds known to target a particular family of kinases known as tyrosine kinases. To some of these compounds, the researchers added a single chlorine atom which effectively served as a brake to keep the atropisomer from spinning around, locking the molecule into either a right-handed or a left-handed version.
When the researchers screened both the modified and unmodified versions against their target kinases, they found major differences in which kinases the different versions inhibited. The unmodified compound was like a shotgun blast, inhibiting a broad range of kinases. But the locked-in right-handed and left-handed versions were choosier.
“Just by locking them into one or another atropisomeric configuration, not only were they more selective, but they inhibited different kinases,” Gustafson explained.
If drug makers incorporated this technique into their early drug discovery process, he said, it would help identify which version of an atropisomeric compound actually targets the kinase they want to target, cutting the potential for side effects and helping to usher drugs past strict regulatory hurdles and into the hands of waiting patients.

