The Rise of 3D Cell Culture and in vitro Model Systems for Drug Discovery and Toxicology

Complete the form below to unlock access to ALL audio articles.
Sarah Holme
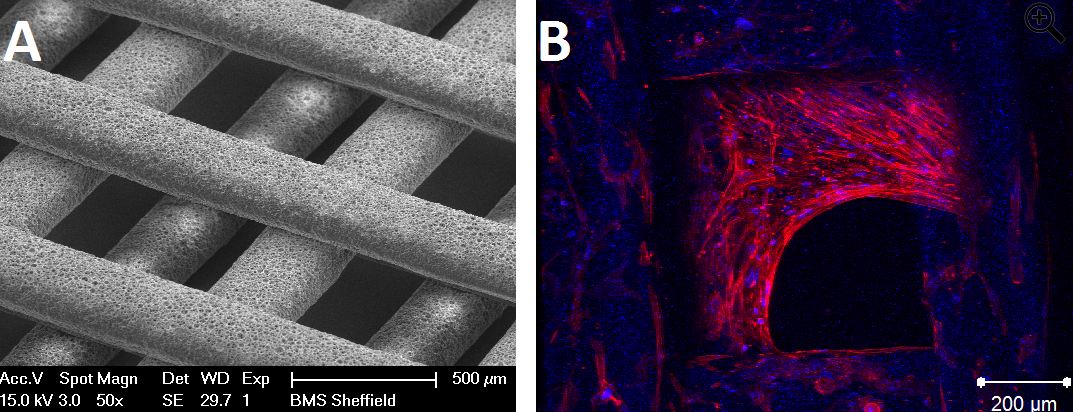
(A) Scanning Electron microscopy image of 3D printed polyHIPE scaffold and (B) confocal image of human Mesenchymal Stem Cell growth on the 3D printed polyHIPE scaffold (blue: cell nuclei, red: actin filaments). Photo courtesy of Dr Frederik Claeyssens, University of Sheffield.
Introduction
3D cell culture is set to dominate in vitro research into human disease and development, in particular new drug discovery and toxicology. There have been huge advances in the past ten years in increasing the complexity and sophistication of 3D in vitro models developed in the lab. In parallel we have witnessed the growth of a sector of companies offering a wide variety of cell types, apparatus, methodology and expertise for 3D cell culture as well as ready-to-use model systems for testing.
The application of 3D cell culture now ranges from basic cell biology research using simple, static 3D monocultures through to complex multi-cellular ‘tissues’ and systems for the perfusion of media around the culture that mimic the in vivo equivalents histologically and, increasingly so, functionally. Cell culture has become so advanced that many researchers are now focussing on culturing systems that mimic whole organs; microfluidics is being used increasingly as the technology becomes more accessible.
Selected Vendors

The novel RAFT™ System creates 3D cell cultures inside a high-density collagen scaffold that more closely mimics the natural environment of cells. An important advantage of the system is its capability of developing complex in vitro models, especially those where a multi-layer co-culture setup has proven difficult to achieve via alternative methods.
" In combination with Lonza’s primary cells and media products, the RAFT™ System allows researchers to delve deeper into cellular biology in pursuit of insight and discovery."
Lubna Hussain, Senior Product Manager for Lonza Bioscience Solutions
Overview of 3D cell culture technology
Human primary material, tissue or cells taken directly from a patient, offers the most functionally equivalent starting material for cell culture but it is often hard to obtain. Human stem cells, in particular human induced pluripotent stem cells (iPSCs), offer the chance to culture and analyse many different human cell types. Stem cells can now routinely be derived from something as straightforward as skin and blood by reprogramming (Takahashi et al., 2007), differentiated and cultured into complex 3D, multi-cellular models mimicking regions such as the heart, liver and even the blood-brain barrier.
The structure of tissues is often comprised of discrete layers of distinct cell types. Growing different cell types together in 3D enables users to recreate such tissue structures in vitro. Co-cultures allow recreating and monitoring of intercellular communication, cell migration dynamics and stimulation of cell function and differentiation.
A 3D cell culture system requires a way to establish 3D growth instead of the flat growth that normally occurs over the surface of the culture vessel. In each case the common goal is to replicate the spatial organisation of a group of cells or tissue in vitro. Scientists typically use hydrogels, suspended cell aggregates or scaffolds to achieve this. Scaffolds can be inert, biodegradable or functionalised, for example with peptides such as binding proteins. Which 3D support is selected is dictated by the biology under investigation and the application.
Professor Stefan Przyborski developed the 3D cell culture scaffold material Alvetex to overcome the challenges he experienced whilst developing in vitro tissue models for his own research. He founded the company Reinnervate in 2002 in order to commercialise Alvetex and to equip the research community with the tools, reagents and methodology they need to set up and run 3D cell culture in the lab (www.reinnervate.com).
‘Alvetex is a porous polystyrene membrane that enables cultured cells to retain their natural 3D architecture and prevent cells from flattening and forming two dimensional (2D) monolayers as experienced in conventional cell culture flasks and Petri dishes’.
‘Much of our understanding of cell biology and the molecular mechanisms that control cell growth, differentiation and function in health and disease is a result of studying cells cultured on flat polystyrene substrates in flasks and dishes. However, within such environments, tissue architecture is lost, cell-cell interactions are reduced, and cells adapt abnormally to their two-dimensional (2D) surroundings by flattening and altering their gene transcription, protein translation and functional phenotype. In contrast, three- dimensional (3D) cultures more closely resemble cells in real tissues and as a consequence show significantly enhanced structure and function (Knight & Przyborski 2014).

Alvetex is now used for many different applications; for example a recent study reported on the 3D reconstruction of a human airway model to study infections (Marrazzo et al. 2016). Professor Przyborski has published an excellent review article on the subject of 3D cell culture (Knight & Przyborski, 2014) and has written a book which aims to be a user’s guide to 3D cell culture technology. The book, which is due to be released later this year, will contain tuition and information from suppliers of 3D cell culture technology to showcase what is available, where to access it and how widely it can be applied.
3D cell culture for drug discovery and toxicology
A lot of time and money is invested by drug development companies to find new target compounds and to analyse the safety and efficacy of these new drugs in the drug development pipeline. In this pipeline, in vitro model systems using human cell and tissue culture are key analysis tools.
Despite decades of experience of designing safe and effective drug development pipelines, the processes are still disconnected, slow and prone to failure. Animal models are used but ultimately they are not human, and there are ethical issues involved with their use. The UK government has leant its support to the move to non-animal technology with its ‘Non-animal technologies roadmap for the UK (drawn up and published by Innovate UK, the UK’s innovation agency). This includes vision for the use of ‘relevant non-animal technologies include complex 3D tissue models, organ-on chips, stem cell platforms, in silico tools and cell imaging approaches’ across a range of timeframes stretching to 2030’. The NC3Rs (www.nc3rs.org.uk) is a UK-based scientific organisation which is actively involved in this process and is dedicated to replacing, refining and reducing the use of animals in research and testing (the 3Rs). The 3Rs principle emphasises the importance of developing alternative approaches in scientific research, which not only avoid the use of animals, but more closely reflects human biology for more reliable data.
In vitro models are needed particularly during the early stages of drug discovery, target compound identification and toxicity testing. 2D cell culture has been instrumental in in vitro drug development for many years, and the techniques employed in their application are well established, sophisticated and informative.
However, companies are now looking to add 3D cell culture assays to increase the predicative accuracy of their target screens. They want to see the real effects of these molecules in a model that replicates the in vivo scenario as closely as possible. Even though currently it is more difficult and more expensive to perform routine cell culture analysis, the data produced is highly valuable in both the short and long term for drug discovery pipelines.
It is a massive jump to move away from the validated and qualified assays already in place that are used in high-throughput in order to incorporate new ones. Companies are overcoming this hurdle by validating new platforms with a small number of molecules which have already been identified and characterised. There are numerous companies that exist to perform this function who have the 3D systems already set up and perform testing of compounds.
In addition, commercially available sources of primary cells or iPSCs, such as those offered by Axol Bioscience, are now helping scientists that develop in vitro models to achieve experimental consistency, reduce batch errors and improve reproducibility across a range of applications including 3D culture.
OrganDots – an organotypic 3D tissue culture platform for research and drug development
Professor Lars Sundstrom from the Elizabeth Blackwell Institute for Health Research, Bristol University, and his collaborator Professor Luc Stoppini of the University of Applied Sciences HES-SO and SCAHT Western Switzerland, in Geneva have created an organotypic 3D tissue culture platform for drug development called OrganDots (Sundstrom et al., 2012). OrganDots are 3D micro tissues that are formed using an air-liquid interface culture system. Stem cells or primary tissue are obtained and dissociated into individual cells, these cells are plated out into wells containing small, semi-permeable membranes and then cultured to produce reconstituted multi-cellular dots that mimic the organ tissue they originated from. Organotypic cultures grown in this way have been demonstrated to have much better viability than 2D cultures.
‘2D cultured cells only last for a limited time; for drug discovery and toxicity testing you need a sustainable system that you can interact with for longer’. Professor Sundstrom
In a recent study from Sundstrom and his collaborators (Rowe et al., 2013) directly compared the growth of human foetal hepatocytes with a conventional 2D sandwich culture versus the air liquid interface membrane 3D culture. In the study the 3D cultures were still alive after 28 days and showed evidence of self-organisation.
Another example of the application of OrganDots is the generation of CardioDots from enriched embryoid bodies. After 2-4 weeks of culturing, CardioDots beat spontaneously with a large and easily measurable frequency and amplitude. CardioDots provide a key system for in vitro toxicology testing as the models are responsive to sodium channel blockers, calcium channel blockers and also potassium channel blockers which are key elements of toxicity testing.
Sundstrom and his collaborators have also recently developed a CNS model using 3D cell culture. There is a particular need for robust CNS in vitro models as most do not work in high throughput. The group was able to form a stimulatable sandwich culture like a ‘mini-brain’ that can be used effectively as a surrogate animal epilepsy model, to allow testing for convulsive effects of test compounds.
Work is now being pursued in Stoppini’s Lab on developing Organ-on-a-chip technology, which is the convergence of 3D cell culture of multiple cell types with microfluidics, and Professor Stoppini is at the forefront of this work combining OrganDots with microfluidic perfusion systems.
A 3D in vitro bone model for the study of osteoporosis
Dr Frederik Claeyssens’ and his group at the University of Sheffield are developing novel materials and structures for 3D cell culture and tissue engineering. Their work has applications in nerve regeneration, corneal repair and more recently in developing bone tissue engineering scaffolds for 3D in vitro models to study osteoporosis. Bone cells are cultured and mineralised on an acrylate-based polyHIPE (polymerised High Internal Phase Emulsions) scaffold and then used to study elements of osteoporosis, such as effects of oestrogen withdrawal (Owen et al., 2016).
HIPEs are typically emulsions of water droplets surrounded by very thin films of a suitable hydrophobic monomer. Under set conditions these are polymerised to form a polyHIPE and then dried – generating a scaffold material. The scaffolds produced have a tensile strength that can be tuned from highly elastic to brittle via selection of the monomers for production of the polyHIPEs. The material/manufacturing process allows for tight control and tuning of porosity and connectivity. The scaffolds are coated with plasma to aid cell attachment and growth. Styrene-based polyHIPEs are the base material used for the Alvetex scaffolds produced by Reinnervate.
In Claeyssens’ work, these emulsions are used as resins for laser-based 3D printing of the scaffolds (Johnson et al., 2013). Via this manufacturing route scaffolds can be produced that are inherently microporous, but with a macroscopic structure resulting from the 3D printing process. These 3D printed foams can be subsequently used as high surface area substrates for cell culture. In collaboration with Dr Reilly (University of Sheffield), human Mesenchymal Stem Cells are grown onto these scaffolds. These un-differentiated stem cells are then differentiated into osteoblasts using medium with selected differentiation growth factors. After 15 days of culture cell growth is seen throughout the scaffold including growth into the scaffold, observed using confocal 3D microscopy. Interestingly, initial results indicate that these polyHIPE generated scaffolds and substrates support not just osteoblasts but also osteocyte-specific growth, something very difficult to achieve in vitro.
The group are using these model systems to study the effect of certain drugs on osteoblast and osteoclast activity. The group are also looking at using bone cell cultures on polyHIPE microspheres for use as injectable scaffolds for treatment of bone lesions.
Current challenges in 3D cell culture
Although 3D cell culture has come a very long way it is far from perfect, and still requires more research and mainstreaming. The future may lie in the challenges now being faced.
“Additional challenges facing 3D culture are cell death and/or uncontrolled cell changes in the more complex (heterotypic) 3D cell microenvironment. Some 3D matrices such as hydrogels are organic, and organic impurities may be introduced into the culture system leading to batch variation and inconsistent results. Synthetics such as non-degradable porous polystyrene (PS) and polycaprolactone (PCL) may overcome these issues. However, these are not without their own limitations such as the ability to interrogate the functional state of the cell by impeding immunofluorescent staining, luminescence or absorbance readouts.
‘Bench-scale’ 3D cell culture systems are evolving and being adapted or scaled-up for automated or high-throughput screening applications. Microfluidics are of increasing importance in this regard. Their use can reduce cell shear stress and aid the transmission of gas, nutrients and cell waste products while maintaining physiological function (e.g. beating cardiomyocyte spheroids ) and ensuring the cells remain amenable to analytical assessment e.g. calcium signalling, contractility, electro-physiology. 3D culture is also shaping current technologies used to monitor tumour growth in cell invasion assays and being used in therapeutics to deliver hydrogel encapsulated cells to the patient. Dr Paul Bello, Axol Bioscience

Full thickness human epidermis model generated using Primary Human Keratinocytes (Axol Bioscience) cultured at
What might be possible with 3D cell culture in the future?
Improvement to Anti-viral drug development
‘There is a high percentage of patient morbidity attributable to secondary viral infection (most notably norovirus, rotavirus) for which we have no effective treatment. Previously, these clinical viral strains have been recalcitrant in 2D cell culture however, it is possible to propagate these in 3D host cells. Hans Clever is pioneering exciting work into the LGR5+ ‘stem cell’ that is able to derive intestinal, brain, pulmonary, pancreatic and other 3D organoids. This opens up avenues for studying viral biology or etiology not only at the bench, but also in scale-up ‘3D’ cell-based anti-viral drug discovery and toxicity testing’. Dr Paul Bello, Axol Bioscience
Increasing complexity
‘In the future we will see increasing complexity in the tissue constructs created. Although it is now possible to layer and / or co-culture several cell types together the systems are still quite simple’. The introduction of perfusion to cell culture systems will bring improvements. ‘Cells and tissues are usually surrounded and perfused due to nearby capillary bed and vasculature and this influences the tissue environment. We are starting to include other cell types beyond the starting co-culture cells. Take the example of skin, as well as the epidermis, dermis and basement membrane, in the future researchers are working to introduce more cell types such as immune cells, melanocytes and endothelial cells’. Professor Stefan Przyborski, Reinnervate
Increased throughput
‘In the future 3D cell culture is likely to become more high-throughput by making the systems smaller, more robust and more reproducible. This will be particularly attractive to larger scale drug discovery pipelines. There will also be advances in technology developed in parallel to support what is possible with 3D cell culture. This will be down to engineers and those who can adapt analytical approaches to in vitro cell / tissue assays. For example, the field of microscopy has developed around imaging cells as a 2D mono layer and the question is now how can we image the 3D tissue systems being generated. Microscopes have not traditionally been designed to do that’ Professor Stefan Przyborski, Reinnervate
The quality and diversity of research now being carried out using 3D in vitro cell culture models is staggering. Although there is still work to be done translating lab scale model systems into robust, replicatable high-throughput systems for routine analysis we can expect the validation and use of a greater range of assays and improved predictive accuracy for drug development and toxicology in the near future.
Sarah Holme is a freelance writer living in Cambridge, UK.
References
Hill, D. S., Robinson, N. D., Caley, M. P., Chen, M., O'Toole, E. A., Armstrong, J. L., ... & Lovat, P. E. (2015). A Novel Fully Humanized 3D Skin Equivalent to Model Early Melanoma Invasion. Molecular cancer therapeutics, 14(11), 2665-2673.
Johnson, D. W., Sherborne, C., Didsbury, M. P., Pateman, C., Cameron, N. R., & Claeyssens, F. (2013). Macrostructuring of Emulsion‐templated Porous Polymers by 3D Laser Patterning. Advanced Materials, 25(23), 3178-3181.
Knight, E., & Przyborski, S. (2015). Advances in 3D cell culture technologies enabling tissue‐like structures to be created in vitro. Journal of anatomy, 227(6), 746-756.
Marrazzo, P., Maccari, S., Taddei, A., Bevan, L., Telford, J., Soriani, M., & Pezzicoli, A. (2016). 3D Reconstruction of the Human Airway Mucosa In Vitro as an Experimental Model to Study NTHi Infections. PloS one, 11(4), e0153985.
Owen, R., Sherborne, C., Paterson, T., Green, N. H., Reilly, G. C., & Claeyssens, F. (2016). Emulsion templated scaffolds with tunable mechanical properties for bone tissue engineering. Journal of the mechanical behavior of biomedical materials, 54, 159-172.
Sundstrom, L., Biggs, T., Laskowski, A., & Stoppini, L. (2012). OrganDots–an organotypic 3D tissue culture platform for drug development. Expert opinion on drug discovery, 7(6), 525-534.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861-872.

