Advances in PCR Diagnostics for Cancer and Medical Mycology

Complete the form below to unlock access to ALL audio articles.
The field of molecular biology would not have advanced to where it is now, if not for Kary Mullis and his Nobel prize-winning polymerase chain reaction (PCR) – an essential technology for the detection and analysis of nucleic acids. Since its introduction to the scientific world in 1983, PCR has become the go-to method for amplifying specific sequences of DNA (and sometimes RNA) for a variety of purposes. Whether it is used for gene-mapping, DNA fingerprinting, detecting bacterial/viral infections, or studying genetic disorders, PCR is usually at the crux of many genomic applications.
For molecular biologists, the allure of PCR lies in its sensitivity and specificity. Most quantitative, real-time PCR assays available nowadays are more sensitive and more specific than plate-based immunoassays – they could detect down to a single nucleic acid molecule.[i]
Digital PCR (dPCR), for example, partitions one sample into a large number of micro-wells, or droplets, and the reaction is carried out in each well individually. This provides a collection of yes/no results and a more reliable and precise quantification. Digital PCR techniques are extremely useful for detecting variations such as copy number variants and point mutations. Also, dPCR has been shown to demonstrate high reproducibility, even across multiple laboratories without calibration.[ii] It overcomes the problems of background noise and relative measurements seen with qPCR (digital PCR allows absolute quantification; no known standards are needed), and enables high-throughput analysis.[iii]
From detecting cancer to microbial infections, and now even fungal infections, PCR has become the gold standard of analyzing gene expression. In this article, we explore how PCR is used as a novel diagnostic strategy to identify clinically important yeast species, as well as to detect mutations that could guide therapeutic decisions for lung cancer patients.
PCR-Powered Liquid Biopsy Can Guide Targeted Cancer Therapy
As the director of the Center for Genetics (CGEN) in São Paulo, Brazil, and a medical oncogeneticist, Dr. Jose Claudio Casali-da-Rocha knows firsthand what a difference wait time can make in cancer treatment. Unlike tissue biopsy, the wait times for liquid biopsy are much less, leading to shorter median times to treatment decision and earlier treatment start times.[iv] The availability of biomarker data early in the treatment process can help oncologists review cases more efficiently and sort out the best treatment options.
But the most important aspect of a liquid biopsy test is the sensitivity – which comes from the underlying technology that detects and amplifies the DNA. According to Casali-da-Rocha, digital PCR is the most practical method for clinical DNA analysis, followed by deep sequencing.
“From our experience, droplet digital PCR (ddPCR) is the most sensitive and fastest test for studying somatic mutations, either in tissue or by liquid biopsy, when you need to analyze a few, known mutations,” says Casali-da-Rocha. “We can accurately and rapidly analyze cell-free circulating DNA with high sensitivity, at a low cost. We’ve found that it makes a better alternative to NGS methods for routinely detecting important biomarkers and genetic variations related to non-small cell lung cancer (NSCLC) that can help determine targeted therapy in these patients.”
Casali-da-Rocha and his team analyzed 38 patients with advanced NSCLC, taking liquid biopsy samples from various sources and performed ddPCR assays to detect genomic alterations.[v] “We used ddPCR for the liquid biopsy of EGFR mutations, including resistant mutations like T790M,” explains Casali-da-Rocha. ddPCR assays are available for a multitude of hotspot alterations across several actionable genes that drive the progression of NSCLC, including EGFR (mutations), ALK (fusion, mutations), ROS1 (fusion), BRAF (mutations), and KRAS (mutations). “We were able to identify meaningful genomic alterations in many patients, where the results were decisive for the indication or the change of a targeted therapy.”
Looking to the future, Casali-da-Rocha points out that proximal liquid biopsy could be a good option for the analysis of CNS, urine, saliva, sperm, and other bodily fluids extracted from patients. As for his institute’s plans for implementing ddPCR-based liquid biopsy, Casali-da-Rocha says, “we want to introduce liquid biopsy at the diagnosis level, before surgery or any treatment, so mutational markers can be better compared to the baseline levels.”
A Novel Multiplex PCR Assay for Identifying Yeast Species
Real-time PCR is a well-established analysis method in medical microbiology, especially for viral and bacterial infections. However, the application of real-time PCR in medical mycology has remained challenging.
Amir Arastehfar, a PhD Student at Westerdijk Fungal Biodiversity Institute in Utrecht, The Netherlands, is nearing the end of his doctoral research which involves studying yeast species, among other things. During his research work with PI, Dr. Teun Boekhout, Arastehfar set out to develop PCR assays that can identify the most prevalent and important yeast species causing infection in humans. “Due to the low number of fungal cells in circulation and the rigidity of their cell wall, using PCR was problematic because it requires an extensive and efficient DNA extraction protocol,” explains Arastehfar. “As a result, some communities, such as Fungal PCR Initiatives (FPCRI) were established to improve the DNA extraction from clinical samples and to design sensitive and specific real-time PCR assays.”
Although culture is the gold standard technique in medical mycology, it does not have sufficient sensitivity, according to Arastehfar. “For example, there might be non-viable fungal cells in circulation not detectable by culture, but easily captured and detected by real-time PCR,” he notes.
Arastehfar and Boekhout were particularly interested in building an affordable and reliable testing approach that could be deployed in developing countries, where there is no (or limited) access to sensitive means of detection, such as MALDI-TOF mass spectrometry or Sanger sequencing. “The World Health Organization has suggested PCR as a good alternative approach for developing countries,” says Arastehfar. “Our PCR is much more specific, sensitive, and rapid when compared to phenotypic assays for which identification requires an array of experiments that almost take 48-72 hours.”
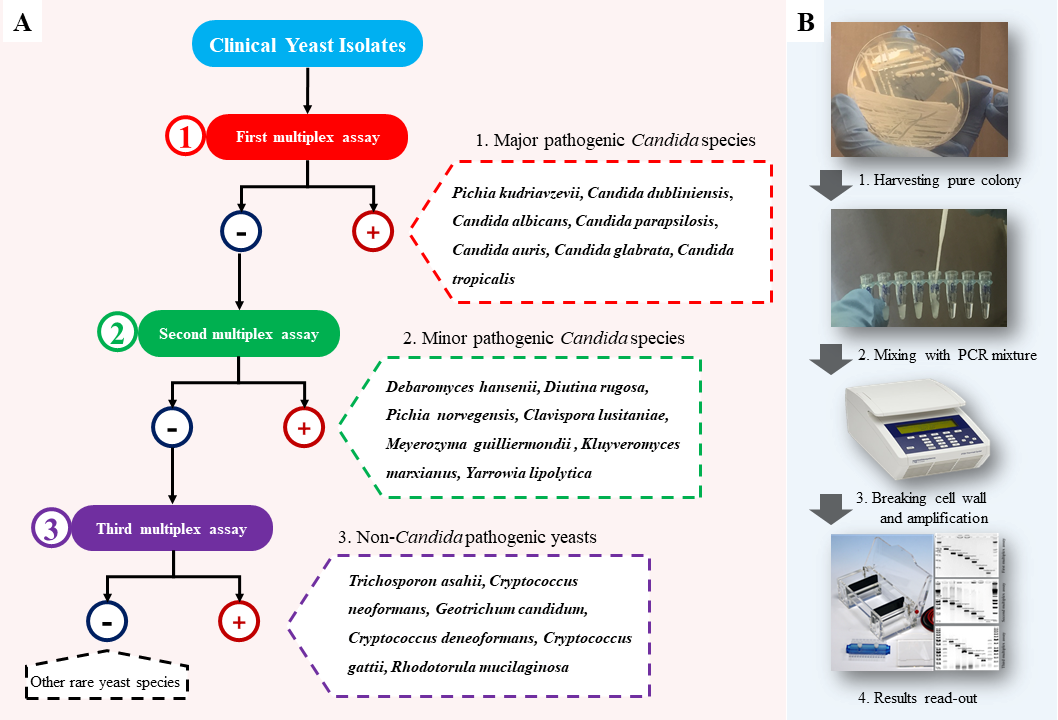
The Iranian and Dutch scientists’ YEAST PANEL multiplex PCR identifies 21 target species (accounting for 95% of the yeast-driven infections) in three multiplex PCR reactions.[vi] The first PCR reaction identifies the most prevalent Candida species, including the multidrug-resistant species, Candida auris. If an isolate is not identified in the first PCR reaction, it can be subjected to the second PCR reaction identifying less prevalent Candida species. Isolates with negative identification using the first and the second PCR reactions are then applied to the third PCR reaction, mostly identifying the basidiomycetous yeast species. (Figure. 1) “Each species in each PCR reaction has a specific and determined length when applied on gel electrophoresis, which is well differentiable from the other species,” notes Arastehfar.
He adds that this approach shortens the turn-around time from 48-72 hours to just a few hours, saves the huge cost associated with biochemical assays, and allows reporting more reliable epidemiological data for developing countries.

Figure 1. The workflow of YEAST PANEL. A) The identification strategy used. If the first multiplex PCR is negative, the yeast colony is subjected to the second multiplex PCR. If the result from the second multiplex PCR is negative, a third multiplex PCR is carried out. If the third multiplex PCR is negative, it could be another yeast species not covered by this multiplex PCR test. B) Two to 3 small colonies are mixed with the PCR master mix followed by running the PCR program. Results are visualized on the gel, and the banding patterns are compared with the respective controls. (Source: Diagnostic Microbiology and Infectious Disease, 2019)
Future Outlook for PCR in Medical Mycology
Although the research world is currently witnessing a shift in the epidemiology of infectious yeast species, the application of real-time PCR in medical mycology still is in its infancy.
Arastehfar and Boekhout hope that, in the near future, efficient DNA extraction methods and highly sensitive and specific real-time PCR assays will accelerate the field. “We have established other multiplex PCR assays supplementing our YEAST PANEL multiplex PCR,” concludes Boekhout. “In the near future, we will evaluate the efficacy of our PCR with clinically obtained blood cultures, urine, and biopsies. We are confident that the high degree of specificity and sensitivity of our PCR when applied on positive blood cultures will aid in choosing an appropriate antifungal drug and lowering mortality rate as a result.”
References:
[i] Milbury, C. A., Zhong, Q., Lin, J. et al. Determining lower limits of detection of digital PCR assays for cancer-related gene mutations. Biomolecular Detection and Quantification, 2014, 1, 8-22.
[ii] Whale, A. S., Devonshire, A. S., Karlin-Neumann, G. et al. International Interlaboratory Digital PCR Study Demonstrating High Reproducibility for the Measurement of a Rare Sequence Variant. Analytical Chemistry, 2017, 89, 1724-1733.
[iii] Schmittgen, T.D., Lee, E.J., Jiang, J. High-Throughput Real-Time PCR. Molecular Beacons: Signalling Nucleic Acid Probes, Methods, and Protocols. Methods in Molecular Biology, 2008, vol 429. Humana Press, 89-98.
[iv] Lim, C., Tsao, M.S., Le, L.W., et al. Biomarker testing and time to treatment decision in patients with advanced non-small cell lung cancer. Annals of Oncology, 2015, 26(7),1415–21.
[v] Murad, A. M., and Rocha, J. C. C. A preliminary single institution experience with droplet digital polymerase chain reaction (dd-PCR) liquid biopsy (LB) for therapeutic decision in advanced solid tumors: Personal precision oncology clinic and genetic diagnostics, Brazil. Journal of Clinical Oncology, 2019, 37, e14613-e14613.
[vi] Arastehfar, A., Fang, W., Boekhout, T. et al. YEAST PANEL multiplex PCR for identification of clinically important yeast species: stepwise diagnostic strategy, useful for developing countries. Diagnostic Microbiology and Infectious Disease, 2019, 93, 112-119.