Pittcon 2016: Accelerating Innovation And Enhancing Productivity With Technology

Complete the form below to unlock access to ALL audio articles.
Helen Gillespie
Informatics was a key focus alongside laboratory equipment and chemical analyses during the Pittsburgh Conference (Pittcon) this year. Held at the Georgia World Congress Center in Atlanta, GA from March 6-10, Pittcon delivered more Informatics sessions than in previous years, with an examination of not just LIMS but data management, data analysis and method development.
The emphasis continues to be on utilizing technology to accelerate innovation and enhance productivity. On the exhibit floor, industry vendors offered a variety of new software and systems to achieve these goals. Interestingly, based on some of the Pittcon presentations, a number of organizations still use manual checklists and spreadsheets to manage lab data or are holding fast to familiar Excel spreadsheets. A growing tsunami of regulations will make it extremely difficult to manage lab data, reviews and signatures this way. Hence, there is a significant opportunity for both vendors and users alike to revisit how to manage lab data.
Collaboration and Method Development
Selected Vendors

Waters highlighted their NuGenesis Lab Management System (LMS) that combines data, workflow and sample management capabilities to support the entire product lifecycle from discovery through manufacturing. The user-centric platform encompasses NuGenesis SDMS, compliance-ready data repository, NuGenesis ELN, a flexible analytical electronic laboratory notebook, and NuGenesis Sample Management.
"Our direction at Waters is to address the three pillars of pain that companies experience by delivering out-of-the-box off-the-shelf tools that reduce complexity, are easy to configure and install, and are easy to qualify so that they solve business problems in the lab."
Dr Rohit Khanna, Senior Vice President of Applied Technology
More Information

ACD/Spectrus is a multi-technique, vendor agnostic platform for chemical and analytical knowledge management. It uniquely captures live data with human interpretations, in chemical context. Chemically intelligent algorithms help scientists extract insights from data for faster decision-making. Workflows may be tailored with automation and solutions may be integrated with existing informatics systems.
"The Spectrus Platform is helping customer organisations effectively capture and share analytical intelligence for the future while helping answer questions the experiments were intended to address."
Sanji Bhal, Director of Marketing & Communications
More Information
Collaboration and method development were examined in depth this year. Academia is certainly enjoying the benefits of technology. Waters® Corporation invited Dr Jessica Prenni, Director of the Proteomics and Metabolomics Facility at Colorado State University and member of Waters' Centers of Innovation program, to discuss some of the collaborative research her team is engaged in. Dr Prenni highlighted the research on biomarker discovery that aims to create method development for dealing with disease diagnosis and treatment. She emphasized that science is evolving and becoming more collaborative, and that project management software is vital to managing the ~60-70 parallel projects that use the university’s mass spectrometers (MS). During Pittcon, Waters announced additional features for their Empower 3 chromatography data software (CDS) which supports MS data acquisition quality and peak tracking capabilities at Colorado State University.
Dr Rohit Khanna, Waters’ Senior Vice President of Applied Technology, pointed out that research labs continue to move toward increased productivity and improved screening and safety, and that the utilization of better data management with informatics tools is one of the ways to accomplish these goals. “Our direction at Waters is to address the three pillars of pain that companies experience by delivering out-of-the-box off-the-shelf tools that reduce complexity, are easy to configure and install, and are easy to qualify so that they solve business problems in the lab,” he said.
Waters highlighted their NuGenesis Lab Management System (LMS) that combines data, workflow and sample management capabilities to support the entire product lifecycle from discovery through manufacturing. The user-centric platform encompasses NuGenesis SDMS, compliance-ready data repository, NuGenesis ELN, a flexible analytical electronic laboratory notebook, and NuGenesis Sample Management.

Many new instruments were introduced during the conference, most with capabilities and features that can enhance productivity and expedite analyses. This is great, but the data needs to be easy to extract, examine and share via an informatics solution. Increasingly those informatics solutions need to share data across an integrated informatics platform, not be locked into a solution silo. Data should be accessible via the CDS, ELN, LIMS and/or LMS.
Dr. Khanna pointed out that method transfer is growing in importance, and thus the informatics platforms need to be able to cope with the variety of method coming in. Waters’ Ian King, Senior Vice President of Instrument Technology, confirmed this, saying ”It’s no longer a software challenge, but a method challenge.”
Oral Sessions Provided Numerous Case Studies
The oral session LIMSLive@Pittcon covered not only online cloud-based LIMS, but also featured many other aspects of LIMS, from selection to implementation to migration. Advanced Technology Laboratories’ (ATL) Vice President of Sales & Marketing, Dr. Christine Paszko, kicked off the session with a presentation based on her popular webinar: Where Do We Start – a Roadmap to LIMS Success, and walked attendees through the six stages of a Needs Assessment plan. She discussed what needs to happen and why all six phases are important to successfully transition to the new LIMS.
All of the subsequent case studies were about or given by ATL’s customers. In one case study, Alan Serrero, Water Quality Coordinator for the Gwennett County Water Resources Lab discussed how they went about their LIMS Needs Assessment. Gwennett County is a suburb of Atlanta, Georgia with a population of 850,000 with three wastewater and two drinking water plants that are served by a single lab. Prior to their LIMS implementation all data was managed with paper checklists and Excel spreadsheets. Raw data was maintained in Excel and reports were printed in Word. However, when two labs were consolidated into one, the data seemed to expand exponentially. They decided to implement a LIMS to reduce transcription errors, make data collection easier, view historical data quickly and generate QC charts. Unfortunately, they didn’t know much about LIMS or what LIMS capabilities were. ATL performed the needs assessment for Gwennett County and installed the LIMS. While pleased with ATL’s solution, Serrero admits that they should have started with the needs assessment before reviewing LIMS systems, rather than having a LIMS vendor walk them through the process prior to implementation. Even so, Serrero said that their LIMS is a good fit and ATL did a good job educating them about what a LIMS can do and how to make it work for them.
In another case study, Keith Keesee, Technical Manager for the Oklahoma Department of Agriculture, discussed issues they dealt with while replacing their aging in-house developed LIMS with a commercial solution. With labs for general chemistry, inorganic chemistry and pesticide analysis, including two microbiology labs, the department considers their LIMS to be mission-critical due to the significant amount of data that’s generated. The first commercial off the shelf (COTS) LIMS they implemented did not go well, neither did the second customized version, wasting five years and $1.2M before the ATL Sample Master LIMS was implemented. Keesee said that they are very satisfied with this solution but a budget shortfall has meant they lost personnel. However, despite the reduced staff numbers, the LIMS has helped them increase efficiency as it has streamlined tasks that were previously performed on paper.
The session closed with a presentation by Dr Maria de la Cantera, Laboratory Manager for the City of Clearwater, Florida, who focused on what it has meant for the labs to have a LIMS. The City of Clearwater’s labs manage the quality of the drinking water and wastewater for ~110K residents, performing ~55K test annually on ~18-20K samples. There are three water treatment plants supplied by 32 freshwater and 12 brackish water wells, two reverse osmosis plants and three advanced wastewater treatment plants. Quality assurance and quality control tests comprise a significant amount of their workload. The LIMS enabled their facilities to go from manual spreadsheets to a digital system, erasing the continuous nightmare of transcription errors and data entered into the wrong place. The greater efficiency, data accuracy and fast report turnaround time have been well received. Dr de la Cantera pointed out that the secure web portal has enabled the data security paper does not, as well as removed the confusion of who has performed which task, eliminating task repetition. In all cases, ATL has proven to be a good fit for these organizations
Informatics for Externalization
Wednesday offered a variety of informatics sessions, including ANIML Data Standards, LIMS-No One Size Fits All, and Big Data in Analytical Sciences: Challenges and Solutions. In addition, Thermo Scientific hosted an in-booth seminar on Innovation Through Laboratory Informatics. Presentations were given by academics pharmaceutical companies, industry consortiums, consultants as well as informatics vendors. Perhaps the most in-depth presentations were to be found in the ANIML workshop and Big Data session where specific details were examined by the presenters, who drilled down into particular challenges and how their organizations solved these challenges. The LIMS session focused on larger issues such as the time savings that can be achieved through a LIMS deployment.
ACD/Labs’ Dr Graham McGibbon, Manager of Strategic Partnerships and Scientific Solutions, gave an interesting presentation on Informatics for Externalization where he observed that, “Pharmaceutical R&D will increasingly rely on external sources for data and information,” emphasizing that there is a real demand for informatics to take this into account.
Dr McGibbon pointed out that challenges in data sharing and collaboration are already apparent throughout most chemically involved R&D industries and are being exacerbated by a trend towards outsourcing core and non-core scientific activities. He predicted that as externalization and research virtualization evolve and business critical tasks of library synthesis, process chemistry, metabolism, toxicology, and intermediates manufacturing are included, the demand for information accessibility will intensify.
Dr McGibbon suggested that a standardized way to collaborate systematically and efficiently via accessing share data would ensure that data is managed in a way that enables data mining for the purpose of identifying raw materials, impurities, metabolites and other chemical ingredients. He said that collaborative workspaces enable creating analytical knowledge packages that can have 'live' analytical data, metadata, and chemistry information independent of instrument source for seamless sharing. He went on to explain that increasingly, external users will be populating internal information through what he termed, “The de-evolution of informatics.”
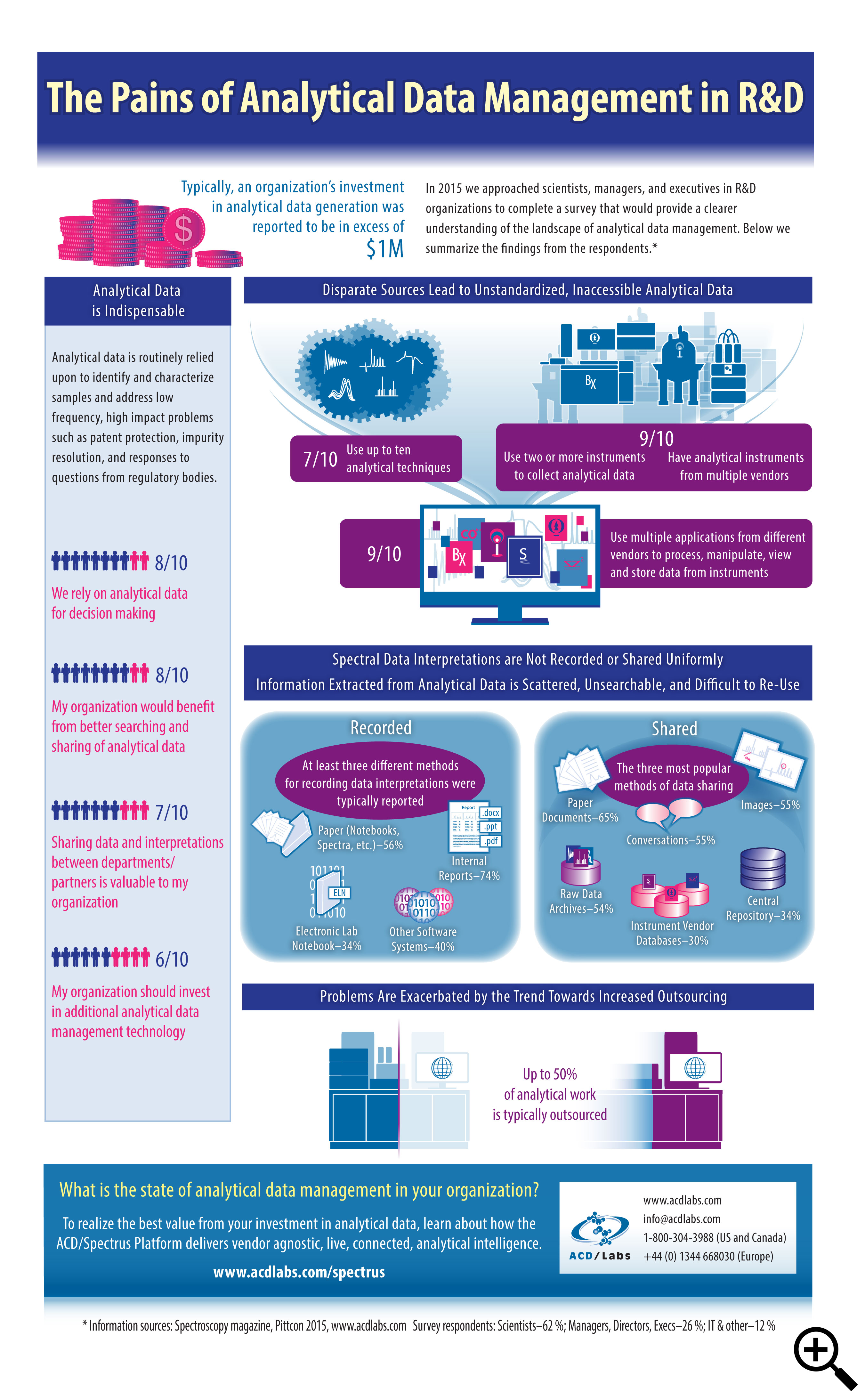
ACD/Labs’ infographic “The Pains of Analytical Data Management in R&D” describes the results of a survey they conducted in 2015 to examine the role of analytical data in research organizations. The results emphasize the complexity of data sources, the difficulties of accessing data from (typically) those disconnected sources, the importance of that data for decision-making, how the data is recorded and shared, and of course the cost of managing that data. The trend toward increased outsourcing is only exacerbating the challenges.
Howard Rosenberg, Principal Industry Consultant for CSOLS, gave a well balanced presentation on LIMS implementation and whether to do a phased approach or all at once. He discussed the pros and cons of each and how to choose the right approach for your organization. The way to stay sane during the project is to plan, plan, plan!
Thermo Scientific’s Trish Meeks, Director of Product Marketing, discussed the various informatics tools available, where they fit into the data management hierarchy, and the benefits of an integrated platform. As a vendor presentation, of course Thermo’s solutions were highlighted, but the information provided applies to any set of informatics tools. The key is to determine what capabilities your informatics system would need to deliver – such as ISO 17025 support – and ensure that the system chosen addresses all those needs. A thorough needs assessment can be vital to a LIMS implementation adoption and its eventual success. Most of the case studies presented during Pittcon highlighted the importance of an accurate needs assessment to ensure that the lab’s requirements are met. The stumbles and missteps occurred when the needs were inadequately mapped out to ensure that the LIMS chosen was the best fit for the lab.
The Big Data sessions explored the specifics of experiment findings, the conclusions, the instruments used and the techniques that delivered the best results. Collaboration with academia was mentioned several times, echoing Dr McGibbon’s presentation earlier in the day concerning the increasing reliance on external users and external data to support in-house research. Method development was a major topic of discussion for the organizations presenting their latest research findings.
Interestingly, many of these organizations rely on paper-based or aging in-house solutions, which is a bit mystifying in this digital age. Although change can be very disruptive, particularly when implementing software, once the learning curve is over, the benefits far outweigh the aggravation.
Certainly consumer technology is driving higher expectations and change in the workplace. Hence the move toward easier to use systems and the ability to accommodate collaboration with a wide variety of different users both internal and external. There’s too much to do and too little time to not take advantage of every opportunity available, so this trend will continue and will hopefully be discussed again at next year’s Pittcon which will be held in Chicago, IL from March 5-9, 2017.
Helen Gillespie is Technology Networks' Informatics Editor

