ICP-MS — ICP-MS Instrumentation, ICP-MS Analysis, Strengths and Limitations
Inductively coupled plasma mass spectrometry can be tailored to meet the demands of a wide variety of industries.

Complete the form below to unlock access to ALL audio articles.
Contents
How do you analyze ICP-MS data and what does it tell you?
Strengths and limitations of ICP-MS
Expanding analytical abilities - MC-ICP-MS and LA-ICP-MS
What is ICP-MS?
Inductively coupled plasma mass spectrometry (ICP-MS) is a flexible chemical analysis method that can be tailored to meet the demands of a wide variety of industries including:- Natural resource exploration
- Petrochemicals
- Environmental (including soil analysis)
- Food and beverage (including wine and drinking water analysis and pesticide screening)
- Regulatory
- Medical
- Pharmaceutical
- Materials and metallurgy
- Nuclear
- Nanotechnology
In its simplest form, ICP-MS takes a pre-prepared liquid containing the analyte, pumps it through a nebulizer to create an aerosol which is introduced into an argon gas plasma. The high temperature of the plasma (~5500-6500 K) is sufficient to atomize and ionize almost all elements, including those with the highest ionization potentials. The analyte ions thus produced can be steered with electrostatic ion optic elements into a mass spectrometer where the ions can be separated into their mass to charge (m/z) ratio and detected. Since ionization is close to 100% efficient for most elements, the counts detected for an elemental ion is representative of its concentration in the analyte.
Introduced in 1983, the method has matured from an instrument with a single detector and a quadrupole mass spectrometer to today where instruments are available with time-of-flight (TOF) mass spectrometers allowing the measurement of the entire mass spectrum in parallel, to single- and double-focusing magnetic sector instruments equipped with single or multicollector detection systems allowing high precision isotope ratio measurements.1
The method of sample pre-preparation has concurrently matured from the initial step of simple acid digestion to today where laser ablation (LA) is commonly combined with ICP-MS for spatially resolved analysis and elemental mapping of solid samples. Furthermore, separation techniques have been coupled to ICP-MS to permit the measurement of molecules, and in particular biomolecules. The ionization process would typically destroy all molecular information from the sample, but this problem has been largely conquered by introducing an element of sample selectivity through pairing separation techniques (e.g., liquid chromatography (LC), gas chromatography (GC), capillary electrophoresis (CE), gel electrophoresis (GE)) with ICP-MS.2,3 The separation technique can isolate out all the species containing the element of interest and sequentially forward them on to the ICP-MS system for detection.
How does ICP-MS work?
A schematic diagram showing the various components of an ICP-MS system is illustrated in Figure 1. Liquid samples are introduced into the nebulizer by a peristaltic pump or self-aspiration where they form an aerosol of fine droplets. Not all nebulizers are the same, and the type chosen is based on factors such as the viscosity, volume and cleanliness of the sample to be analyzed.
Figure 1: Schematic showing main components of an ICP-MS system described in more detail in text. Credit: Technology Networks.
The fine droplets that are created by the nebulizer are passed through a spray chamber before entering the plasma. Different types are again available, but the function remains the same: to allow a high number of the small droplets to enter the plasma while discriminating against the larger droplets which can create analytical issues if permitted into the plasma.
The argon plasma generated in the ICP reaches temperatures of between 5500-6500 K and is generated by passing argon gas through concentric quartz tubes (commonly referred to as the ICP torch) that are contained at one end within a radio frequency (RF) coil. Energy supplied to the coil by an RF generator couples with the argon gas to produce the plasma. As the liquid droplets enter the high temperature plasma, they are converted to the gaseous state. As they absorb more energy, they will eventually release an electron to form a single, positively charged ion.
The interface region where the ions produced by the plasma are introduced to the mass spectrometer presents an engineering challenge. In the first instance, the torch region reaches temperatures of ~6000K, the other side of the interface remains at room temperature. Secondly, the torch will have been necessarily backfilled with the Ar gas required to generate the plasma, while the mass spectrometer will be under high vacuum conditions. Each manufacturer will have different solutions, but two or more cone structures (lenses) are used to prevent a wide divergence of the ions as they enter a region of high vacuum, and to focus them into the collision cell or directly into the mass spectrometer.
Ions will not be the only species exiting the plasma – neutral atoms and photons will be present. Photons can give rise to false ion counts so it is important that they are removed from the path of ions. There are variations on a theme as to how the different manufacturers deal with this issue, but a common solution is to place some form of lens element that will selectively bend only the ions into the quadrupole mass spectrometer.
Most modern instruments will have a “universal” or “reaction/collision” cell located between the ion optic elements and the mass spectrometer to help reduce the problem of mass interferences. This occurs when two ions, an elemental ion (e.g. 56Fe+) and a molecular ion that may result from the interaction between the sample matrix and the Ar gas in the plasma (e.g. 40Ar16O+), have ostensibly the same m/z value – 56 amu (atomic mass units). Many of these interferences will be difficult to separate based only on the mass resolving power of the mass spectrometer. Unless this mass interference is eliminated, the resultant measurement will have a high background and lower detection limit directly attributable to the 40Ar16O+ or other interfering ions.
In collision mode, the cell is backfilled with a partial pressure of inert gas, and both the elemental and molecular ions will lose some of their kinetic energy through collisions with the inert gas atoms as they travel through the cell. The likelihood of such collisions will be greater for the much larger 40Ar16O+, so when it reaches the end of the cell, it will have lost far more kinetic energy. By placing a kinetic energy band pass filter at this point, the two types of ions can be effectively separated by their differences in kinetic energy, and only 56Fe+ will continue its journey into the quadrupole mass spectrometer. However, the 56Fe+ intensity will have been slightly cut as well by the energy filter, albeit at a much smaller proportion. Nonetheless, the detection limit will be adversely affected.
In a reaction cell, the inert gas atoms are replaced by a reactive gaseous species. The rationale is that the introduced gas will react with the interfering species to produce a neutral species that can no longer be influenced by the electrostatic fields of the ion optics or the quadrupole. It will be effectively filtered out. The analyte ion is not affected, and thus compared to the collision cell it is a more powerful method for eliminating mass interferences. However, care needs to be taken to ensure that no “new” mass interferences are generated during this process.
Quadrupole mass spectrometers are most commonly found in ICP-MS instruments, although others based on magnetic sectors and time-of-flight are available. Quadrupole mass spectrometers measure one mass at a time, with the RF voltage and direct current (DC) offset voltage set to allow only ions of a single specific m/z value oscillate through the region between the four poles that make up the spectrometer. Ions of other masses will collide with the rods and be eliminated. The RF and DC voltages can be serially scanned to allow for detection through the desired m/z range in an analysis. Depending upon the configuration, magnetic sector mass spectrometers work in a similar fashion, except the magnetic field is scanned to bend the trajectory of ions within the desired m/z range through to the detectors.
As mass spectrometers can have significant length through which the ion must travel to achieve mass separation, it is absolutely imperative that this region is under high vacuum. Otherwise, analyte ions could collide with gas molecules leading to possible charge exchange reactions and decreasing overall sensitivity and increasing unwanted mass interferences.
How do you analyze ICP-MS data and what does it tell you?
Data in ICP-MS is generally analyzed either quantitatively or semi-quantitatively, as isotope ratio measurements or in isotope dilution analyses.
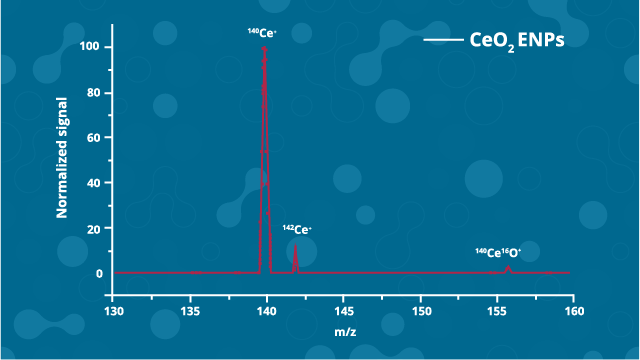 Figure 2: Example of an ICP-MS mass spectra. Credit: Technology Networks.
Figure 2: Example of an ICP-MS mass spectra. Credit: Technology Networks.
With this knowledge, full quantitative analyses can be planned with appropriate standards, and instrument operation protocols adjusted in the event of identified potential mass interference problems. Quantitative analysis proceeds by deriving calibration curves that will convert measured analyte counts to a concentration. To create this curve, a set of reference standards that have a verified concentration of the analyte are measured under the exact same instrumental conditions as the unknown sample. For these types of analyses the quadrupole RF and DC voltage settings or the magnetic sector magnetic fields need only be set for the m/z values of interest. There is no need to scan through the entire mass range. Finally, the method of isotope dilution is a means of achieving the highest quantitative accuracy. For example, if the analyte is 56Fe+, the sample could be diluted by adding a known quantity of 57Fe, a stable isotope which has a natural abundance of 0.29% and the same chemical and physical properties of 56Fe. It therefore acts as an internal standard. By measuring the isotope ratio 57Fe/56Fe, and since the amount of added 57Fe is known, the concentration of 56Fe can be calculated. The beauty of this method is that the analysis, standardization and quantification is all done in a single experiment, and the end result is arrived at by a ratio, so any instrumental effects will be ruled out.
Strengths and limitations of ICP-MS
The strengths of ICP-MS include its ability to analyze almost all elements in the periodic table at concentrations in the low ng/L range with a high dynamic range. High sample throughput is another forte which is particularly important in industrial applications. Low sample volumes, often with reasonably simple preparation methods, are generally sufficient to produce the required results. The method is also capable of distinguishing isotopes, both stable and radioactive, for high precision measurement of isotope ratios. Finally, as discussed previously, ICP-MS is highly suitable as a selective detector in hyphenated methods using some form of separation method to allow determination of analyte species.
Limitations include the relatively high cost of the equipment which requires a high level of staff proficiency. Mass interferences can also be a problem but there are various ways that these effects can be minimized or eliminated, as discussed.
Common problems with ICP-MS
Some of the most common problems related to ICP-MS usually have at their origin problems at the nebulizer or the cone ion optics elements. Inconsistencies in the ion count rates and precision repeatedly measured from the same sample are likely to be observed if these elements are dirty or damaged. Problems with the peristaltic pump may also lead to similar issues.
As the technique is so sensitive, it is also prone to memory or carryover effects whereby the detection limits for an analyte may be artificially increased by its residual presence in the instrument resulting from previous analysis of samples or calibration standards with high concentrations of that same element.
Mass interferences are always an issue, and solutions to help mitigate their effect have been described. Though collision and reaction cells in single quadrupole systems are effective at eliminating elemental and molecular ion mass interferences, they are far less effective when it comes to eliminating isobaric mass interferences (two elemental ions with the same isotopic mass e.g. 58Ni and 58Fe) or interferences at m/z caused by doubly charged ions (z = 2; eg.56Fe++ and 28Si+). Separating these types of interferences typically requires triple quadrupole systems.
Finally, lab components, from reagents to water to glassware, must be impeccably clean and of high purity in order to prevent unintentional sample or standard contamination.
Expanding analytical abilities - MC-ICP-MS and LA-ICP-MS
Advances in expanding the capabilities of ICP-MS include the introduction of multicollector (MC) ICP-MS systems and using laser ablation (LA) of solid samples as a means to introduce sample into the ICP-MS system without recourse to some form of digestion in liquid and removing all spatial information.
The MC-ICP-MS systems are found on magnetic sector instruments, whereby the dispersion of the mass separated ions caused by the magnetic field can be focused onto different detectors in a multicollector array. This allows for the steady state analysis of samples with all isotopes measured simultaneously and thus instrumental effects become time invariant. Their biggest applications are in geochronology and cosmochemistry where high precision isotope ratios are required.
LA-ICP-MS is widely used to determine elements directly in solid samples with minimal sample preparation and the spatial distribution of the analyte across the sample (or in specific areas) is required. It enables imaging ICP-MS. The sample surface is irradiated with a high energy laser (e.g. deep UV, 213 nm wavelength) in an ablation chamber or cell purged with Ar. The beam diameter can be adjusted to below 5 µm up to 300 µm and is a first approximation of the spatial resolution of the method. The aerosol produced by the laser interaction with the sample is carried to the ICP in Ar where it is subsequently ionized and analyzed. The beam may be used in spot mode to analyze specific features on the sample, or rastered across the sample to provide image maps of the distribution of trace elements in the sample. Quantification is a bit trickier as matrix effects are more abundant – different materials will interact with the laser beam in different manners. As a result, for the best quantification results, matrix matched standards are preferred.
ICP-MS vs ICP-QQQ
Despite the advances made in reducing mass interferences with reaction and collision cells in ICP-MS, there remain occasions where the desired detection limits are lower than achievable with these cells. Combinations of analyte and sample matrix will exist that produce mass interferences that cannot be resolved with a reaction or collision cell alone. Isobaric interferences and the problem of doubly charged ions mentioned in section 5 are examples. Specifically, doubly charged ions of rare earth elements Nd, Sm and Gd cause interferences at 75As and 78Se, trace elements of importance in the food industry for their toxicity (As, and Se at high levels) and nutritional importance (Se, which is essential for production of selenoproteins). In ICP-QQQ ("triple quad"), an additional quadrupole is placed before the reaction cell. Thus, all ions are effectively mass filtered to within 1 amu before entering the reaction cell. In other words, only ions with e.g. m/z = 75 (As and its mass interferences) enter the cell. This allows the design of specific targeted chemical reactions to take place. In this case, O2 can be used in the reaction cell to produce 75As16O+ (a technique called mass shifting) which can be detected at m/z = 91 and be free of any potential interference from 91Zr as these ions would have already been rejected by the first quadrupole.4
What's the difference between ICP-MS vs ICP-OES?
ICP-MS and ICP-OES (inductively coupled plasma optical emission spectroscopy) are similar in that both techniques introduce samples from a liquid via aspiration or pump into a nebulizer where an aerosol is produced that is injected into an argon plasma. As the name suggests, optical emission spectroscopy does not analyze the m/z of the ionized atoms, but rather measures the wavelength of the characteristic energy emitted from atoms as their electrons fall from a highly excited state back to the ground state.
ICP-OES is a more convenient method to use. The instrumentation is less expensive compared to ICP-MS, it does not require the same level of staff expertise to run, and reagent grade chemicals are usually sufficient for analysis. It also benefits by its ability to look at solutions with higher dissolved solids content that would otherwise present problems to ICP-MS instrumentation. However, the detection limits will not be as low as for ICP-MS.
1. Meermann B, Nischwitz V. ICP-MS for the analysis at the nanoscale - a tutorial review. J Anal At Spectrom. 2018 Sep;33(9). doi: 10.1039/C8JA00037A
2. Bustos ARM. The role of ICP-MS in separation science. Chromatographia. 2020 Feb 17;83(2):145–7. doi: 10.1007/s10337-019-03846-2
3. Xu X, Wang H, Li H, Sun H. Metalloproteomic approaches for matching metals to proteins: The power of inductively coupled plasma mass spectrometry (ICP-MS). Chem Lett. 2020 Jun;49(6):697–704. doi: 10.1246/cl.200155
4. Jackson BP, Liba A, Nelson J. Advantages of reaction cell ICP-MS on doubly charged interferences for arsenic and selenium analysis in foods. J Anal At Spectrom. 2015;30(5):1179–83. doi: 10.1039/c4ja00310a



