A Small Twist in Protein Structure Leads to a Big Reaction
Complete the form below to unlock access to ALL audio articles.
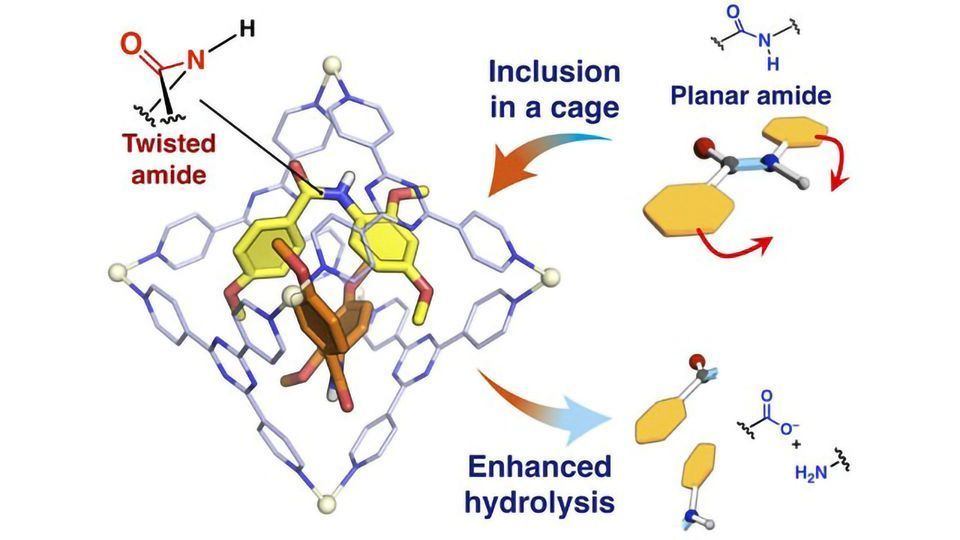
In proteins, amino acids are held together by amide bonds. These bonds are long-lived and are robust against changes in temperature, acidity or alkalinity. Certain medicines make use of reactions involving amide bonds, but the bonds are so strong they actually slow down reactions, impeding the effectiveness of the medicines. Researchers devised a way to modify amide bonds with a twist to their chemical structure that speeds up reactions by 14 times.
The amide bonds that hold amino acids in place to form proteins ordinarily have a flat shape, said to be planar. This planar arrangement is known to give amide bonds their incredible resilience to change. So if a reaction requires a breakdown of amide bonds, it’s going to have a hard time unless it gets some help.
One such reaction is called hydrolysis, a chemical breakdown due to the presence of water. This is used by some medicines, for example antibiotics, within the body. Hydrolysis is also used in the lab as a tool for chemical analysis, amongst other things. So if hydrolysis can be made faster, it could benefit research in these areas. And this is exactly what Professor Makoto Fujita and his team at the Department of Applied Chemistry have done.
“Amide bonds can be hydrolyzed in time given sufficient heat or pH balance, but this can be inefficient or expensive at scale,” said Fujita. “For many years we have fabricated self-assembling molecular cage structures which can confine and modify structures within them. In this case, we confined amide bonds in molecular cages which then applied a slight twist of 34 degrees to their otherwise planar structures. And the results are encouraging.”
What Fujita and his team discovered was that given this slight twist in their structures, amide bonds were able to be hydrolyzed at a much faster rate with certain configurations, increasing their reaction rate by up to 14 times. This could be of great benefit to various medical researchers in the fields of drug development and more besides.
Reference
Takezawa et al. (2020). Enhanced reactivity of twisted amides inside a molecular cage. Nature Chemistry. DOI: https://doi.org/10.1038/s41557-020-0455-y
This article has been republished from the following materials. Note: material may have been edited for length and content. For further information, please contact the cited source.

