New Microscopy Technique Shows Cells’ 3D Ultrastructure in New Detail
Complete the form below to unlock access to ALL audio articles.
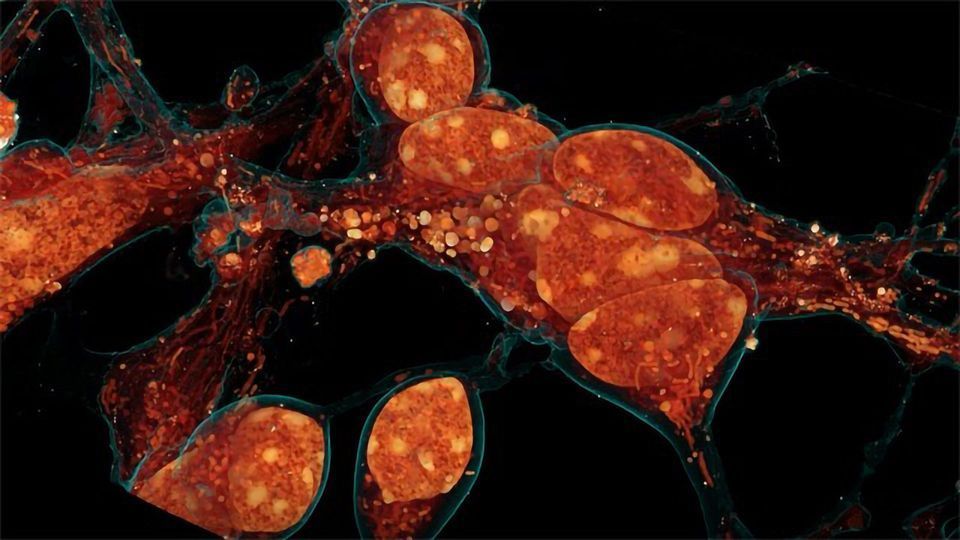
Inside a cell, tentacled vesicles shuttle cargo for sorting. Neighboring neurons cling to one another through a web-like interface. DNA rearranges in the nucleus as stem cells differentiate into neurons. And a new microscopy technique shows it all, in exquisite detail.
The technique, called cryo-SR/EM, melds images captured from electron microscopes and super-resolution light microscopes, resulting in clear, detailed views of the inside of cells – in 3-D.
For years, scientists have probed the microscopic world inside cells, developing new tools to view these basic units of life. But each tool comes with a tradeoff. Light microscopy makes it simple to identify specific cellular structures by tagging them with easy-to-see fluorescent molecules. With the development of super-resolution (SR) fluorescence microscopy, these structures can be viewed with even greater clarity. But fluorescence can reveal only a few of the more than 10,000 proteins in a cell at a given time, making it difficult to understand how these few relate to everything else. Electron microscopy (EM), on the other hand, reveals all cellular structures in high-resolution pictures – but delineating one feature from all others by EM alone can be difficult because the space inside cells is so crowded.
Combining the two techniques gives scientists a clear picture of how specific cellular features relate to their surroundings, says Harald Hess, a senior group leader at the Howard Hughes Medical Institute’s Janelia Research Campus. “This is a very powerful method.”
Janelia Research Scientist David Hoffman and Senior Scientist Gleb Shtengel spearheaded the project under the leadership of Hess and Janelia senior fellow Eric Betzig, an HHMI Investigator at the University of California, Berkeley.
First, the scientists freeze cells under high pressure. That halts the cells’ activity quickly and prevents the formation of ice crystals that can damage cells and disrupt the structures being imaged. Next, the researchers place samples in a cryogenic chamber, where they’re imaged in 3-D by super-resolution fluorescence microscopy at temperatures less than ten degrees above absolute zero. Then, they’re removed, embedded in resin, and imaged in a powerful electron microscope developed by the Hess lab. This scope shoots a beam of ions at the cells’ surface, milling away bit by bit while taking pictures of each newly exposed layer. A computer program then stitches the images together into a 3-D reconstruction.
Finally, researchers overlay the 3-D image data from both microscopes. The result: stunning imagery that reveals cells’ inner details with remarkable clarity.
Below, a few examples of this imagery illustrate how scientists are using the technique. “There’s already been a lot of interest,” says Hess. “There are so many more experiments to do — a whole world of cells out there to study.”
Reference
Hoffman et al. (2020) Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells. Science. DOI: https://doi.org/10.1126/science.aaz5357
This article has been republished from the following materials. Note: material may have been edited for length and content. For further information, please contact the cited source.

