New High-resolution Mass Spectrometry-based Workflow Simplifies Biopharmaceutical Characterization and Quality Control
Thermo Scientific HR Multi-Attribute Method (MAM)
Want to listen to this article for FREE?
Complete the form below to unlock access to ALL audio articles.
Read time: 1 minute
With the launch of a new high-resolution mass spectrometry-based workflow, biopharmaceutical scientists are no longer restricted by the complex series of assays typically required for the characterization and quality control of protein therapeutics.
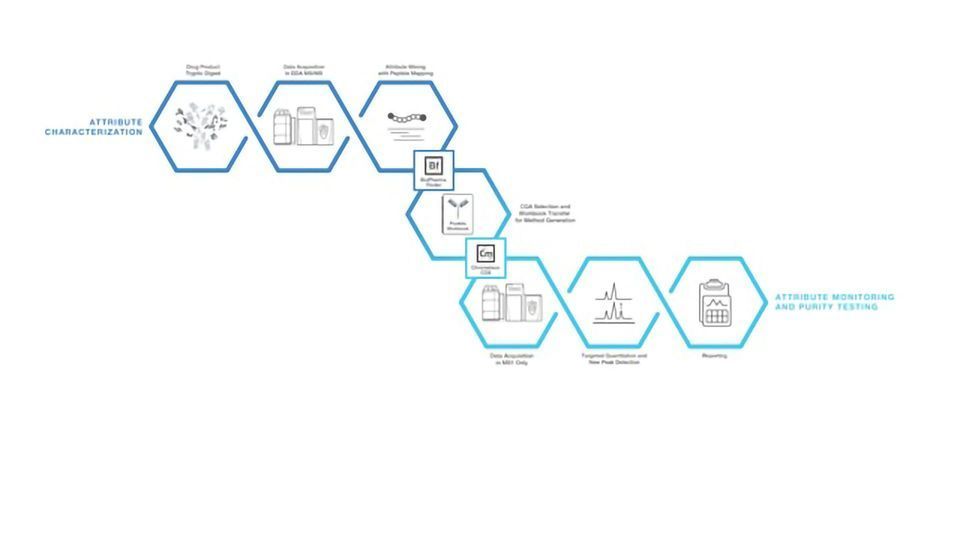
The Thermo Scientific HR Multi-Attribute Method (MAM) meets the demand for a single, high-resolution mass spectrometry-based workflow designed to directly assess the product quality attributes of increasingly complex biotherapeutics. The workflow replaces the complicated sequence of lower-resolution methods that have previously prolonged characterization timelines. Supported by the Thermo Scientific Chromeleon Chromatography Data System (CDS) software, the HR MAM method simplifies and standardizes biotherapeutic characterization throughout the product pipeline, facilitating the transition of new drugs from initial research to development and manufacture.
Thermo Fisher Scientific is showcasing its new workflow during the 67th American Society for Mass Spectrometry (ASMS) Conference on Mass Spectrometry and Allied Topics, being held June 2–6, in the International Ballroom ABCD at the Omni CNN Center Hotel, Atlanta, Georgia.
Richard Rogers, PhD, Just Biotherapeutics, Inc., said, “Powerful Orbitrap technology and efficient search algorithms, like the one implemented in the BioPharma Finder software, allow us to fully leverage the HR MAM in the characterization of our biotherapeutics. One key component is new peak detection, which is essential for using MAM as a QC release method. The HR MAM contains all of the software components for characterizing and releasing biotherapeutics from QC.”
Additionally, companies including Amgen and Pfizer are testing and, in some cases, implementing MAM with Thermo Fisher Scientific products for their analytical processes.
“As a trusted partner of the biopharmaceutical industry, we appreciate that the ultimate goal of drug therapy development and manufacturing is to deliver the highest quality product at the lowest cost and in the shortest timeframe possible,” said Linda De Jesus, vice president and general manager, high performance chromatography solutions, Thermo Fisher Scientific. “With the launch and application of the HR MAM, the characterization and monitoring of biotherapeutics can be standardized across an organization – wherever their manufacturing operations are in the world – to ensure that each batch released is safe and of the highest quality.”
The HR MAM provides biopharmaceutical scientists with:
• A supported, comprehensive workflow from sample analysis to data processing, including new peak detection.
• Method development, SOP management and product quality attribute discovery capabilities through the integration of the Thermo Scientific BioPharma Finder software with Chromeleon CDS software.
• Flexible, 21-CFR part 11 compliant Chromeleon CDS software that supports the transition of development programs from early research to manufacturing and testing.
• High-resolution accurate mass capabilities and quality data to accurately identify peptides and post-translational modifications and allow reliable new peak detection using the Thermo Scientific Q Exactive Plus mass spectrometer.
The Thermo Scientific HR Multi-Attribute Method (MAM) meets the demand for a single, high-resolution mass spectrometry-based workflow designed to directly assess the product quality attributes of increasingly complex biotherapeutics. The workflow replaces the complicated sequence of lower-resolution methods that have previously prolonged characterization timelines. Supported by the Thermo Scientific Chromeleon Chromatography Data System (CDS) software, the HR MAM method simplifies and standardizes biotherapeutic characterization throughout the product pipeline, facilitating the transition of new drugs from initial research to development and manufacture.
Thermo Fisher Scientific is showcasing its new workflow during the 67th American Society for Mass Spectrometry (ASMS) Conference on Mass Spectrometry and Allied Topics, being held June 2–6, in the International Ballroom ABCD at the Omni CNN Center Hotel, Atlanta, Georgia.
Richard Rogers, PhD, Just Biotherapeutics, Inc., said, “Powerful Orbitrap technology and efficient search algorithms, like the one implemented in the BioPharma Finder software, allow us to fully leverage the HR MAM in the characterization of our biotherapeutics. One key component is new peak detection, which is essential for using MAM as a QC release method. The HR MAM contains all of the software components for characterizing and releasing biotherapeutics from QC.”
Additionally, companies including Amgen and Pfizer are testing and, in some cases, implementing MAM with Thermo Fisher Scientific products for their analytical processes.
“As a trusted partner of the biopharmaceutical industry, we appreciate that the ultimate goal of drug therapy development and manufacturing is to deliver the highest quality product at the lowest cost and in the shortest timeframe possible,” said Linda De Jesus, vice president and general manager, high performance chromatography solutions, Thermo Fisher Scientific. “With the launch and application of the HR MAM, the characterization and monitoring of biotherapeutics can be standardized across an organization – wherever their manufacturing operations are in the world – to ensure that each batch released is safe and of the highest quality.”
The HR MAM provides biopharmaceutical scientists with:
• A supported, comprehensive workflow from sample analysis to data processing, including new peak detection.
• Method development, SOP management and product quality attribute discovery capabilities through the integration of the Thermo Scientific BioPharma Finder software with Chromeleon CDS software.
• Flexible, 21-CFR part 11 compliant Chromeleon CDS software that supports the transition of development programs from early research to manufacturing and testing.
• High-resolution accurate mass capabilities and quality data to accurately identify peptides and post-translational modifications and allow reliable new peak detection using the Thermo Scientific Q Exactive Plus mass spectrometer.

