Advanced Data Analytics in Pharma: Going Beyond Patterns to Understand the “Why”

Complete the form below to unlock access to ALL audio articles.
New developments in pharmaceuticals have made new drugs and treatments available, enhancing options and quality of life for patients. Advanced data analytics solutions mean treatments are more effective and affordable, and less intrusive. However, these evolutions will mean major changes in how companies interact with patients and caregivers, and this will necessitate new capabilities for operations and supply chain.
The intelligent supply chain, a development based on these data analytics technologies, is best at demand anticipation and characterization (localization and service levels) by identifying and understanding the patterns influencing it. However, to facilitate the new care options for patients that current developments can offer, it is necessary to go beyond seeing patterns to understand the “why” behind them.
Anticipation tools allow pharmaceutical companies to ensure clinicians have fluid and fast access to their treatments. For new types of treatments, e.g., individualized therapies with “batch of one” drugs, operations should be transformed and new capabilities are needed, such as recruitment of data scientists, sociologists and AI specialists, among others. Companies need to build the skills to adapt their factory and personnel planning to demand in real time, and industrialize their approaches.
Joint development with experienced partners is required, of course, but not sufficient: injecting own business and process expertise into the system is required to understand causal relations influencing demand-and-supply patterns sustainably. The most advanced pharma companies have started to experiment with new forms of analytics to change the way operations are planned and service is delivered, but solutions are not yet at an “industrialized” level. (See figure 1.)
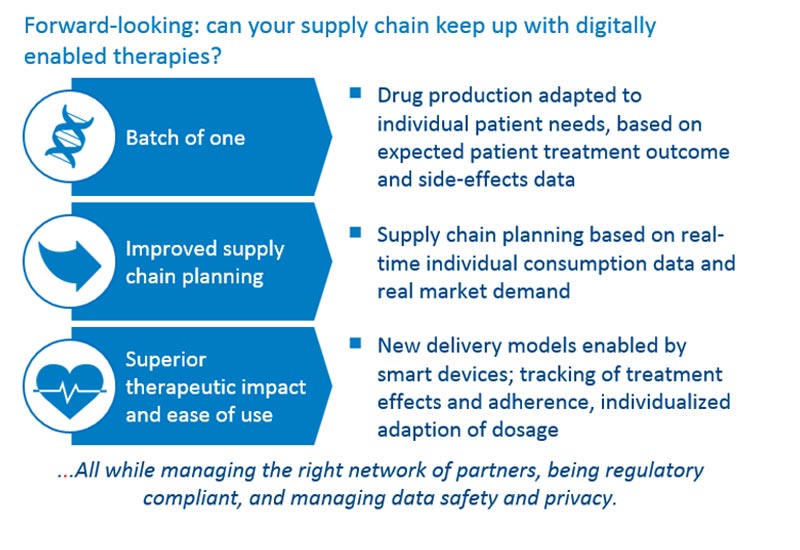 Figure 1. Source: Arthur D. Little
Figure 1. Source: Arthur D. Little
Building confidence in the systems
A prerequisite to mobilizing investments and competences around predictive analytics is to develop confidence in the systems. To move forward in this, many executives face the challenge of navigating through a vast data analytics environment that encompasses multiple concepts. The following practical advice explains how companies can test and leverage existing analytics pilots to outline key causal factors to integrate into the predictive supply chain. (See figure 2.)

Figure 2. Source: Arthur D. Little
First, list and understand pilots. This is to better understand methodologies applied to various pilots, confirm their underlying hypotheses, and identify whether other options are available. Secondly, test pilot methodologies on historical company data, and compare their results to actual history, in order to exclude most of the “false positive” methodologies and identify repeated correlations. These methodologies should be tested in an exhaustive, systematic and automated manner.
Thirdly, qualitatively validate scenarios. At this point, the correlations have proved to be resilient over time; hence, causality can be assumed, but is not yet proven. A large number of “false positives” have already been excluded, and a qualitative assessment of the repeated correlations is the best way to identify meaningful (with explainable links – no random coincidences) and useful causality links (in which the variables can be measured).
Lastly, test for “future-proofness”. Ultimately, the stability of the methodology is pressure-tested, applying “derived laws” in the previous step against all possible future scenarios to evaluate maximum variability and their implications through multi-variate analysis.
Experimenting with data analytics solutions
Leading pharmaceutical companies have begun piloting data analytics solutions in different functional domains, essentially starting with R&D. They then investigate how these foundations can be exploited in other domains.
For example, GlaxoSmithKline is using a machine learning-based platform to improve pre-clinical candidates’ discovery, in partnership with Exscientia. This technology designs new molecules; assesses them for their potency, selectivity and ability to bind to specific targets; and uses a rapid “design-make-test” cycle to modify drug candidates according to the desired criteria.
Similarly, Pfizer and IBM have partnered to accelerate drug discovery in immuno-oncology thanks to Watson technology. Their collaboration aims to quickly analyze and test hypotheses extracted from large and broad data sources (laboratory and data reports, medical literature).
In a different context, we have seen the use of predictive analytics in production quality management: predicting from the external factors of the production chain which batches will be likely to deviate from the quality requirements and, consequently, significantly improve planning in the downstream part of production.
Conclusion
Pharmaceutical companies are looking into the next paradigm to deliver the promise of new treatments and build superior competitive advantage. With the emergence of data analytics solutions, the transformation from reactive to predictive supply chain brings the potential for a superior patient-and-caregiver experience. However, gaining confidence and know-how in the new predictive models is a prerequisite to the major capability-building effort required. To enable such models, companies need to develop advanced analytical models along with the ability to understand the causes and effects that will make their predictions successful.

