Label-Free Binding Analysis Trends

Complete the form below to unlock access to ALL audio articles.
Introduction
The real-time analysis of biomolecular interactions (e.g. protein:protein, protein:peptide, and protein:small molecule and fragments) are of high importance to researchers across many scientific disciplines. Biomolecular interaction analysis facilitates the understanding of binding mechanisms and kinetics. This information is critical to both clone selection and characterization in the development and production of antibodies and biotherapeutic proteins. It is also a significant component in understanding the affinity of small molecule binding to a therapeutic target during lead optimization.
Although ELISA still remains one of the most widely used methods of investigating binding interactions biosensors-based label-free (LF) detection systems (particularly those based on surface plasmon resonance (SPR) and bio-layer interferometry (BLI) are now the preferred way to perform kinetic characterization and undertake affinity measurements. The number of different LF detection systems offered had risen dramatically in recent years, of particular note has been the increase in systems based around SPR.
In July 2014 HTStec undertook a market survey on label-free binding analysis mainly among research labs in pharma, biotech and academia. The main objectives of this survey were to comprehensively document current experience of and future interest in investigating biomolecular interactions and binding analysis using LF technologies. The study also examined in detail the changing market landscape, application areas and future purchasing plans. This article contains ‘selected findings’ from the HTStec market report, Label-Free Binding Analysis Trends 2014. It is intended to provide the reader with a brief insight into recent market trends. It covers only 10 out of the 31 original questions detailed in the full report. The full published report should be consulted to view the entire dataset, details of the breakdown of the responses for each question, its segmentation and the estimates for the future. Please click here for more information.
Selected Vendors
Calypso CG-MALS system

The Calypso® system characterizes biomolecular interactions from first principles, without labelling or immobilization. Composition-gradient multi-angle light scattering (CG-MALS) directly determines molar mass as a function of concentration and composition, quantifying the binding affinity and absolute molecular stoichiometry of complexes including self- and hetero-association, multi-valent complexes and more.
"The Calypso fully complements other label-free technologies commonly used in structural biology labs, providing rich data, minimal method development, and simple but rigorous analysis."
Daniel Some, Ph.D., Principle Scientist
Pioneer FE with Hit-Selection Software

The Pioneer FE (Fragment Edition) is a Label-Free Surface Plasmon Resonance (SPR) platform with novel, patented OneStep gradient injection technology. Now, optimization of screening workflows can be accomplished with minimal assay development time. The Pioneer FE comes standard with best-in-class, industry proven, hit-selection software.
"Pioneer FE SPR instrumentation optimizes drug discovery workflows by eliminating those candidates that will eventually fail in both discovery and development applications."
Tom Jobe , Chief Operating Officer
XelPleX, Multiplex Label-free Interaction analysis platform

XelPleX is a high-performance and fully automated instrument for the analysis of label-free molecular interactions in a multiplex format. It combines the sensitivity of SPR analysis and the high-throughput of array-based experiments – enabling you to accelerate your research.
"Involved in molecular analysis for over 20 years, I'm proud to share with you the launch of the XelPleX, which is a technological achievement while providing easy access to molecular screening and offering the flexibility to be adapted to a wide range of applications."
Chiraz Frydman, PhD, Product Manager
Areas of work and where binding analysis is used:

Figure 1. Survey respondent's main area of work
The best description of survey respondent’s main area of work in presented in Figure 1. This showed that the main area of work of survey respondents was 32% biopharmaceutical discovery & development. This was followed by 25% small molecule drug discovery & development; 22% academic research; 9% immunodiagnostic development; 5% vaccine development; 4% reagent development; and then 3% other.
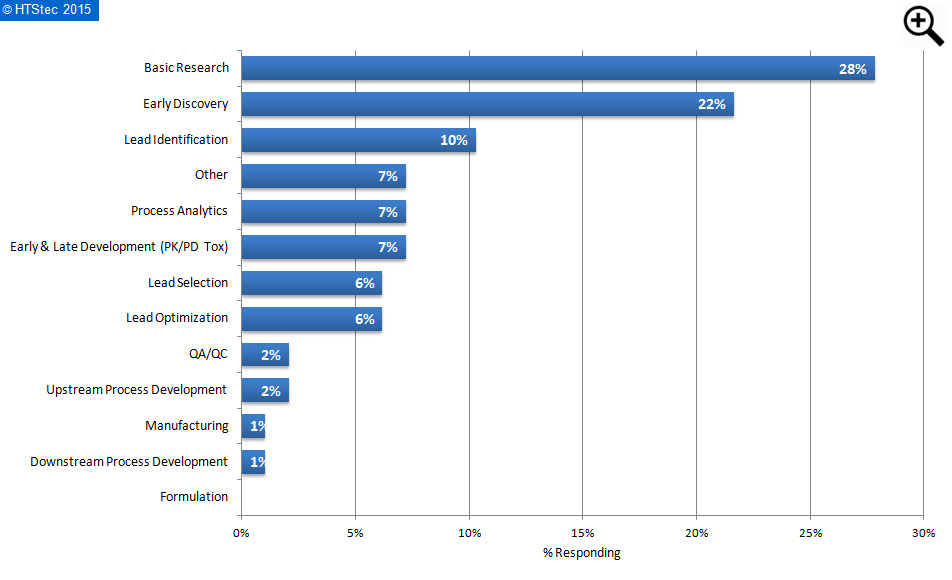
Figure 2. Area where biomolecular binding analysis is used primarily
The area where biomolecular binding analysis is primarily used or preformed is given in Figure 2. This showed that most survey respondents primarily used biomolecular binding analysis in the area of basic research (28% engaged). This was most closely followed by early discovery (22% engaged) and lead identification (10% engaged). All other functions had less than 10% of survey respondents engaged.
Classification of a label-free binding assay:

Figure 3. Classification that most closely describes a label-free binding assay
The classification that most closely describes a label-free binding assay is given in Figure 3. This showed no clear agreement was obtained as to the classification that most closely describes an LF binding assay, as most (41%) survey respondents selected ‘all of the above’. This was followed by real-time binding kinetics method (23% selecting); affinity measurement method (12% selecting); immunoassay method (10% selecting) and then protein interactions monitoring method (9% selecting).
Limitations of label-free assays:

Figure 4. Most common limitation experienced using a LF assay approach
The most common limitation survey respondents have experienced using a label-free assay approach is presented in Figure 4. This showed that lack of detection sensitivity was the most common limitation experienced by survey respondents using an LF approach. This was followed by not fast enough to results; other; and then limited choices of chip surface chemistries. Lack of ready automation was not regarded as a significant limitation by any respondent.
Label-free binding assay applications:
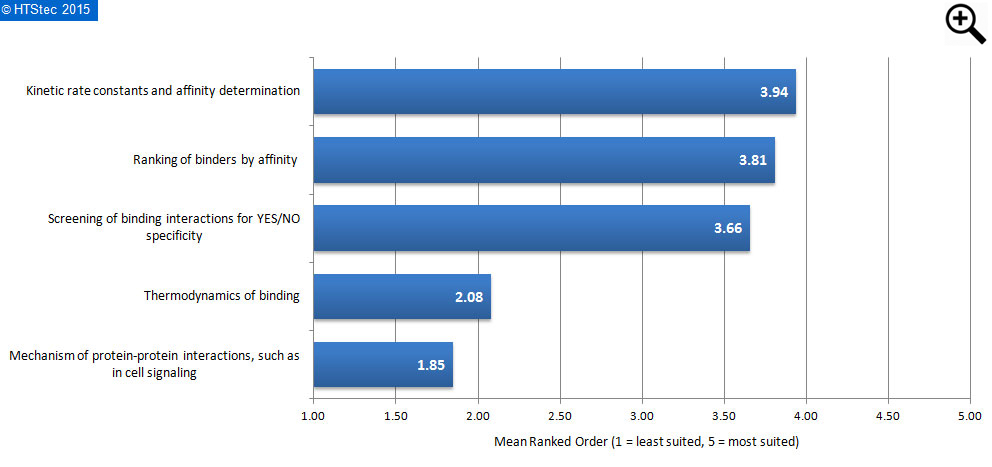
Figure 5. Applications LF binding assays are most suited for
Opinion on the applications that label-free binding assays are most suited for are given in Figure 5. This shows that survey respondents ranked LF bindings assays were most suited for kinetic rate constants and affinity determination. This was very closely followed by both ranking of binders by affinity, and then screening of binding interactions for YES/NO specificity. Mechanism of protein-protein interactions, such as in cell signaling were ranked least suited.
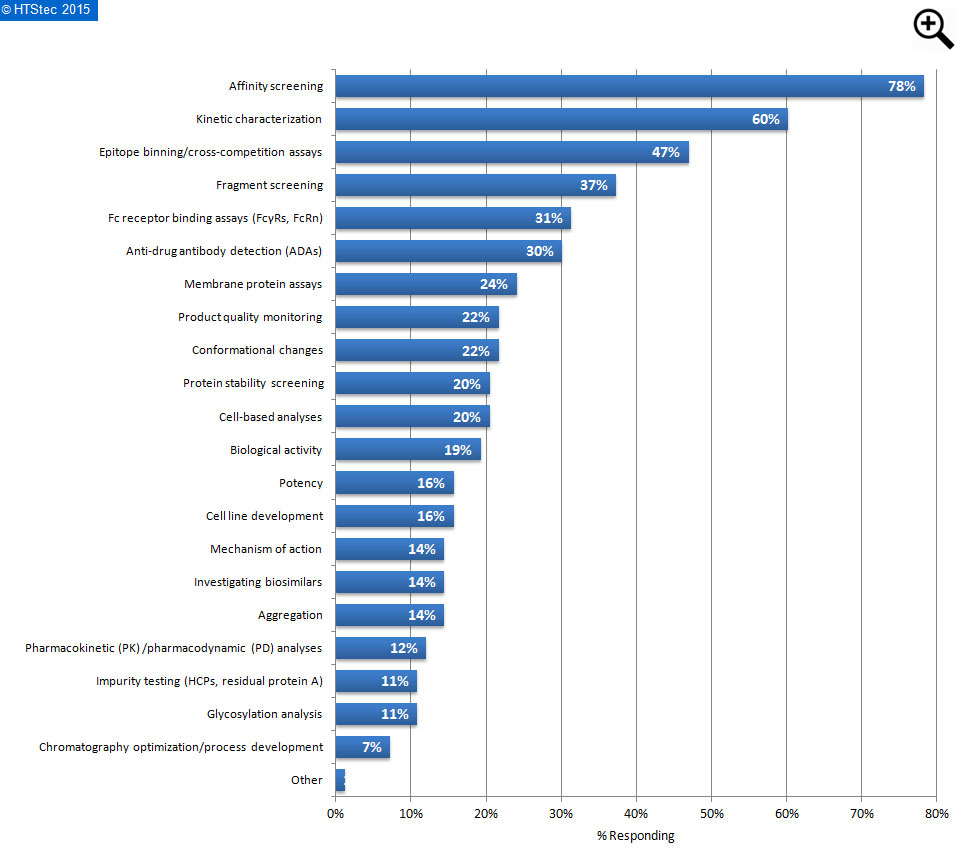
Figure 6. Application areas where respondents expect LF binding analysis to gain popularity
The application areas where survey respondents expect label-free binding analysis to gain most popularity as a valid technological approach within the next few years are detailed in Figure 6. This showed that affinity screening was the most anticipated popular application area of survey respondents. This was followed by kinetic characterization; epitope binning/cross-competition assays; and then fragment screening.
Expanding the use of label-free binding assays:

Figure 7. Processes that would most benefit from the use of LF binding analysis assay technologies
The processes that would benefit most from the expanded use of label-free binding analysis assay technologies are reported in Figure 7. This showed that from an extensive list of processes secondary screening (hit confirmation) was the one that most survey respondents thought would benefit from greater use (49% choosing). This was very closely followed by hits-to-leads (lead optimization) (48% choosing) and characterization of biologics (47% choosing). After these came small molecule screening (HTS); screening of biologics; and discovery of biologics all with 43% choosing. Least benefit was expected for upstream process development and pre-clinical drug safety.
Purchasing new label-free systems:

Figure 8. Important aspects when purchasing a new LF system for binding analysis
The importance given to aspects when purchasing a new LF system for binding analysis are rated in Figure 8. This showed that real-time monitoring/binding kinetics capability was ranked the most important aspect when purchasing a new LF system for binding analysis. This was closely followed by high affinity interactions analysis, and then cell-based and biochemical assay capabilities combination.

Figure 9. Desirable characteristics or attributes that most influence a LF system purchasing decision
The desirable characteristics or attributes of a label-free binding analysis system that would most impact a future purchasing decision are given in Figure 9. In this question survey respondents were allowed to select their top 3 attributes, the results presented are a % of all selections. This showed that system reliability and robustness was the most selected desirable system characteristic when purchasing (17.6% of all selections). This was followed by confidence in data (15.6% of all selections); user-friendly software & improved data analysis tools (15.2% of selections); and only then value for money (with 8.7% of all selections).

Figure 10. Vendors LF binding analysis instruments respondent's are most interest in purchasing*
The vendor survey respondents were most likely to purchase a new label-free instrument from over the next few years are presented in Figure 10. In this question survey respondents were allowed to select their top 2 vendors, the results presented are a % of all choices. This showed that instruments from GE Healthcare/Biacore were of greatest interest (32% choices). This was followed by ForteBio (25% choices), PerkinElmer (7% choices); Bio-Rad and Life Technologies (both 6% of choices) and then Corning (4% choices). All other vendors had less than 4% of choices. The results should NOT be regarded as a true market share projection. It is based on survey respondent's future purchasing preference for vendors instruments, not on their $ sales value.
Conclusions:
The selected findings reported above show that most interest in label-free binding analysis resides in the groups involved in biopharmaceutical and small molecule discovery and development. Apart from basic research, biomolecular binding analysis is primarily used in early discovery and lead identification. Most respondents view a label-free binding assay as a multi-purpose tool, encompassing real time binding, affinity measurement, immunoassay alternative and protein-interaction monitoring method. Lack of sensitivity and speed of acquiring results remain the main limitations of LF approaches. The main applications of LF binding applications were kinetic rate constants and affinity determination and the ranking of binders by affinity. These applications, together with epitope binning/cross-competition assays and fragment screening are the areas where LF binding assays are expected to gain most popularity over the coming years. The processes expect to gain most from the wider use of LF binding assay technology were secondary screening (hit confirmation, hits-to-leads (lead optimization), and the characterization of biologics. The most important aspects where considering the purchase of a new LF system for binding analysis were real-time monitoring/binding kinetics capability and high affinity interactions analysis, together with system reliability and robustness. Interest in purchasing new LF binding analysis systems still remains greatest with the market leaders (GE Healthcare/Biacore and ForteBio). However, there is significant interest in the alternative LF system vendors particularly where they appear to offer some unique capability or support niche applications. Overall the market does not appear to be constrained by value for money or by insufficient availability of label-free instrumentation. The current impact on LF binding analysis on HTS of small molecules is currently limited and could ultimately be surpassed by alternative label-free approaches (high throughput mass spectrometry).
DISCLAIMER: HTStec Limited has exercised due care in compiling and preparing these Selected Findings from its Report, which is based on information submitted by individuals in respondent companies. HTStec Limited has NOT verified the accuracy of this information, nor has it is established respondent’s authority to disclose information to HTStec Limited. HTStec Limited expressly disclaims any and all warranties concerning these Selected Findings including any warranties of mechantability and/or fitness for any particular purpose, and warranties of performance, and any warranty that might otherwise arise from course of dealing or usage of trade. No warranty is either expressed or implied with respect to the use of these Selected Findings. Under no circumstances shall HTStec Limited be liable for incidental, special, indirect, direct or consequential damages or loss of profits, interruption of business, or related expenses that may arise from use of these Selected Findings, including but not limited to those resulting from inaccuracy of the data therein.

