Liquid Chromatography – Including HPLC, UHPLC and LCxLC
How does liquid chromatography (LC) work, how has the technique evolved, and how is LC data interpreted?

Complete the form below to unlock access to ALL audio articles.
Contents
What is liquid chromatography?
How does liquid chromatography work?
The evolution of UHPLC from HPLC
The hyphenation of mass spectrometry to liquid chromatography (LC-MS)
How do you read an LC-MS mass spectrum and what does it tell you?
Taking liquid chromatography into multiple dimensions
Semi-preparative liquid chromatography for purification
Strengths and limitations of liquid chromatography
Liquid chromatography (LC) is a chromatographic technique used to separate and analyze mixtures of chemical components in solution, to determine if a specific component is present or absent and, if present, how much of it is there. Many of us will be familiar with a form of planar LC from our school days, making a black ink mark on filter paper, dipping the end in water and watching the component colors within the ink separate out as the water soaks up the paper. However, the majority of LC used in analytical applications is based around column chromatography, which will be the focus herein.
High performance liquid chromatography (HPLC), as the name would suggest, is the high-performance variant used for high efficiency separations with high chromatographic resolution. Separated components may also be isolated post detection as a means of purification, using a fraction collector. HPLC is available in a variety of different configurations and is used for the separation of dissolved components ranging in molecular weight from semi-volatile small molecules up to large protein biomolecules of several tens of thousands of kilodaltons.
Liquid chromatography is a very popular analytical technique used for many applications including but not limited to:
- Environmental monitoring
- Drinking water analysis
- Food security and quality control
- Air quality analysis
- Chemical industry testing
- Clinical diagnostic including neonatal screening
- Pharmaceutical and biopharmaceutical analysis
- Screening for drugs of abuse
How does liquid chromatography work?
A variety of different system configurations are available for a liquid chromatograph, with the highest efficiency separations being run on ultra-high performance liquid chromatography (UHPLC) instrumentation, this technique was first commercialized in 2004 and was called ultra performance liquid chromatography (UPLC). In short, a multi-component mixture that is soluble in the liquid mobile phase is separated due to the individual components’ unique partitioning between the mobile phase (Figure 1 (1)) and the stationary phase (column) (Figure 1 (3)).
The mobile phase, typically a solvent, is used to transport the sample through the system with the aid of a high-pressure pump (Figure 1 (1)). However, it also plays a critical role in the separation process.
A small volume of sample (1-100 µL) is loaded into a sample loop (Figure 1 (2)), and is then injected into the mobile phase flow by means of a six-port valve and this triggers the start of the chromatographic run. Once the sample has been injected, the mobile phase is pumped through to the column (Figure 1 (3)). A variety of column lengths (30 to 250 mm) and internal diameters (1 to 4.6 mm) are available, packed with stationary phase adsorbent materials of differing activities and particle sizes (1.5 to 10-micron diameter) that together define the column efficiency and selectivity. The column is located in a column oven; at higher temperatures (45 ºC) the viscosity of the mobile phase decreases which increases its linear velocity. This in turn reduces the run time and also improves the chromatographic resolution.
Components in the mixture that have a higher affinity to the mobile phase will migrate through the column quickly with little interaction with the stationary phase. As the band of the component leaves or elutes from the column, the detector (Figure 1 (4)), will give a response that is proportional to the concentration of the component. The time taken between injection and detection is known as the retention time. The retention time for a component will be very specific for a given set of chromatographic conditions and may be compared with that of a standard for identification.
In the case of reversed phase chromatography, less polar analytes will preferentially partition into the non-polar stationary phase and have a longer retention time. The data acquisition system (Figure 1 (5)) records the detector response as a function of retention time in a chromatogram. The peaks recorded in the chromatogram (Figure 2) are usually integrated to determine the peak area which is proportional to the concentration of the component present in the sample.
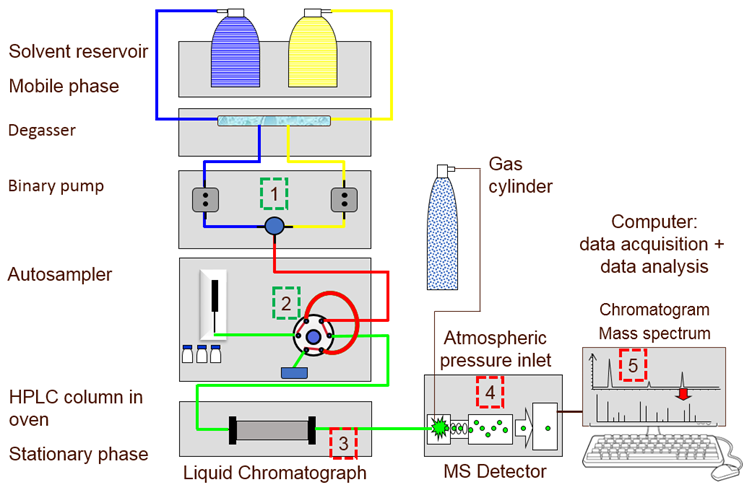
Figure 1: A simplified diagram of a liquid chromatograph hyphenated to a mass spectrometer (LC-MS) showing: (1) binary pump for mobile phase, (2) autosampler 6-port valve and injector loop, (3) column heater with column, (4) mass spectrometer detector, (5) PC. Credit: Anthias Consulting.
After the autosampler injects the sample into the mobile phase the separation process is carried out in the column. The selectivity of the chromatographic system has the largest influence over the chromatographic resolution and should be tailored for the application and components under investigation. The selectivity may be modified by changing the eluotropic strength of the mobile phase (different solvents) or the specific chemical functional groups present in the stationary phase (changing the column type).
 Figure 2: Chromatogram output from an HPLC or LC-MS. Credit: Anthias Consulting
Figure 2: Chromatogram output from an HPLC or LC-MS. Credit: Anthias Consulting
Considering the mobile phase, there are two main modes of operation to choose from when running a liquid chromatograph, namely, isocratic or gradient. An isocratic method will use the same mobile phase composition for the duration of the chromatographic run with no change in selectivity. A gradient method will enable the mobile phase composition to be changed as a function of time, which is usually optimized to either increase the chromatographic resolution or shorten run times.
The chemistry of the stationary phase immobilized within the column influences the selectivity of the technique. Reversed phase HPLC or UHPLC1 is the most popular system configuration and employs a non-polar stationary phase, such as octadecylsilane (ODS or C18), and a polar mobile phase (water/methanol). Other reversed phase stationary phases include octasilane (C8) which is less hydrophobic than C18 with correspondingly shorter retention times for less polar analytes. If a column is used that is functionalized with a phenolic substituent, this will increase the retention of phenolic components due to their increased affinity; like attracts like.
The pH of the mobile phase has a profound effect on the retention times of ionic components, and this should be leveraged during the method development process. Buffers2 may be used to maintain the pH of the mobile phase two units below the pKa of the ionic component which in turn shifts its dissociation equilibrium to the neutral form. The neutral form of a component will be less polar and therefore its retention time can be controlled.
Normal phase chromatography is another LC method that separates analytes based on their polarity and was in fact developed prior to the introduction of reversed phase liquid chromatography but is less popular. The stationary phase is polar in normal phase chromatography3 and the mobile phase is non-polar. This changes the retention characteristics of the system, with non-polar components of the mixture eluting first with the shortest retention time. Polar analytes will have a higher affinity to the stationary phase and elute later with a longer retention time. There are other types of liquid chromatography available including ion chromatography, including ion exchange and ion pair, size exclusion, affinity and the list goes on. With the exception of size exclusion chromatography, which separates analytes based on their size/shape or molecular weight, the other forms of LC mentioned all employ different mobile and stationary phase chemistries. The selectivity and chromatographic resolution attainable for a given set of components to be separated is defined by the stationary and mobile phases employed.
The components, once separated, require detection. The choice of detector is driven by the method goals for the application; a variety of options are available with various degrees of sensitivity, specificity, selectivity and linear dynamic range. The most popular detector is the ultraviolet-visible (UV-Vis) detector that measures the absorbance of light at a specific wavelength. The wavelength is selected based on the lambda max of the component to be analyzed, the detector response is directly proportional to the concentration of that specific component. As the component elutes off the column its concentration within the flow cell of the detector will rise and fall and this in turn is plotted as the chromatographic peak (see Figure 2). The data acquisition rate should be set to acquire at least 20 data points across the peak. As with so many chromatographic techniques, hyphenation to a mass spectrometry system usually offers the best analytical resolution with a wide range of options available.
The length and internal diameter of tubing used to interconnect the various components of an LC system are critical and should be kept to an absolute minimum. Any part of the chromatography system, from the start of the injection loop through to the end of the detector flow cell, that is not the stationary phase does not contribute to an efficient separation. This additional volume within the system is known as the void volume, additional longitudinal diffusion of the separated components within the void will result in a loss of sensitivity and reduced chromatographic resolution.
The evolution of UHPLC from HPLC
The evolution of UHPLC was in part driven by the analyst’s ever-increasing requirements for higher resolution separations of increasingly complex and challenging samples. The major breakthrough that enabled this step change in chromatographic performance was the development of sub-2-micron stationary phase packing material4 with a narrow particle size distribution.
The new particles were manufactured with the same chemical functionality as the commonly available HPLC stationary phases which ensured that the selectivity of the chromatographic system was maintained when using the same mobile phase. The significant performance benefits were realized by the increased efficiency or plate count afforded when using new sub 2-micron packing materials.
There are many advantages to behold when migrating HPLC methods onto a UHPLC system including shorter run times, higher chromatographic resolution, increased sensitivity and less solvent consumption. In order to use the novel UHPLC columns it is necessary to use a pump that can operate at a higher pressure in order to accommodate the increased back pressure exerted by the smaller particles in the column. The detector flow cell also requires upgrading to have a smaller internal volume which is necessary to detect the narrower bands of the components eluting off the column. The data acquisition rate also needs to be increased accordingly to ensure sufficient data points across the peaks.
The hyphenation of mass spectrometry to liquid chromatography (LC-MS)
Mass spectrometry is arguably the best detector that can be hyphenated to a liquid chromatograph due to its high sensitivity, linear dynamic range, selectivity and even specificity when using instrumentation with a very high mass resolving power. The technique of mass spectrometry is used to determine the mass-to-charge ratio (m/z) of a component or analyte. Unlike gas chromatography-mass spectrometry (GC-MS), the hyphenation of an LC system to MS was not easy and took many years to develop. Electrospray ionization (ESI) is the most common ionization technique used in LC-MS today, where the ionization process takes place at atmospheric pressure. It was the development of an atmospheric pressure inlet to the high vacuum required within the mass spectrometry system that was difficult to achieve. Microflow and low-flow LC-MS are proving useful in areas including biomarker detection and biopharmaceutical analysis.
How do you read an LC-MS mass spectrum and what does it tell you?
Electrospray ionization is a very soft ionization technique meaning that there is very little fragmentation observed during the formation of ions. An LC-MS system may be run in either positive ion mode for basic analytes generating protonated molecules [M+H]+, or negative ion mode for acidic analytes generating deprotonated molecules [M-H]-.
It is possible to fragment ions generated by the electrospray process usually through collision induced dissociation (CID) in order to gain more information for characterization or identification of target analytes. CID may be performed in the ion source by changing the potential difference applied to the first sampling or skimmer cone, or, in a collision cell where the ions are accelerated into a collision gas such as argon.
Figure 3 represents a full scan LC-MS acquisition with in-source collision induced dissociation to produce a series of characteristic fragment ions for each separated component of the mixture. The mass-to-charge ratio (m/z) is plotted along the x axis and the intensity or relative abundance of the ion is plotted along the y axis. The z axis on figure 3 represents the retention time of the separated components, each baseline resolved chromatographic peak is analyzed by the mass spectrometer one at a time, key diagnostic fragment ions may be used for identification and target ion confirmation.
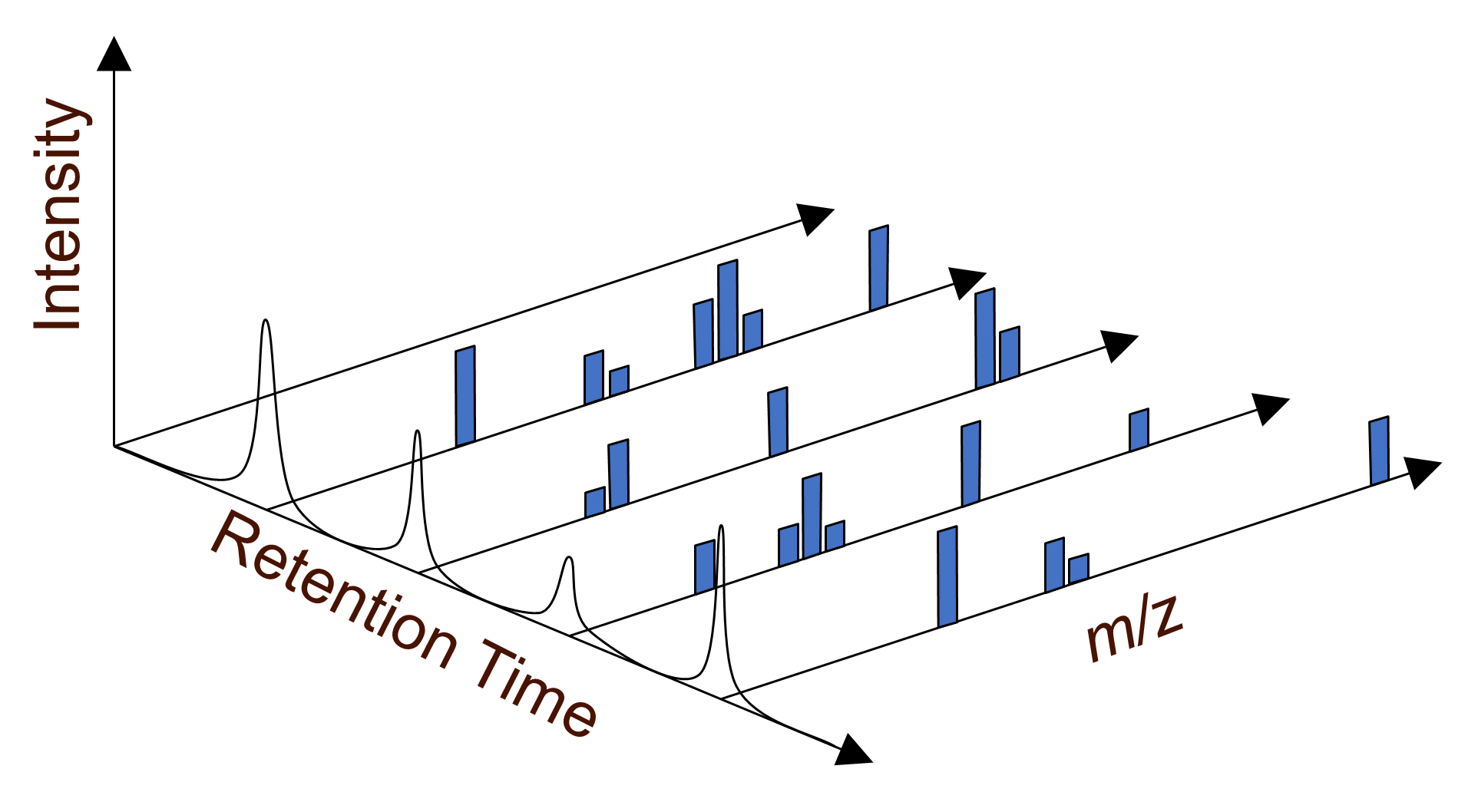
Figure 3: A series of full scan LC-MS mass spectra, MS adds an additional dimension of information. Credit: Anthias Consulting.
Taking liquid chromatography into multiple dimensions
When dealing with complex multi-component mixtures, it may not be possible to baseline resolve each and every component as individual peaks eluting off the column. Method development and optimization can only get you so far, if the chromatographic resolution is not available due to lack of selectivity then it may be necessary to use an additional chromatographic column with an alternative selectivity to separate co-eluting components.
Multi-dimensional chromatography5 enables co-eluting components to be diverted by means of a “heart cut” onto an alternative chromatographic column with a more suitable selectivity, that can resolve the separation into individual eluting components. The system may be set up with a divert valve that simply diverts the co-eluting peaks post detection in the first dimension or column into the second dimension, for subsequent separation and detection with a higher chromatographic resolution. The improved capabilities of multi-dimensional LC has proven useful in many areas including foodomics, food safety and traceability.
Semi-preparative liquid chromatography for purification
By scaling up the dimensions of a liquid chromatography system, using columns with a larger internal diameter running at higher flow rates, it is possible to load more material onto the column. Semi-preparative chromatography6 systems may be loaded with 100’s of milligrams of sample. A fraction collector is then used to collect the chromatographic peaks into separate vials as they elute off the column. The fraction collector is triggered by the detector, which looks for an inflection in the chromatographic baseline indicating the start of a peak, and the peaks of the separated components are collected as pure fractions. The isolated fractions can then be subjected to additional analytical techniques that complement mass spectrometry, such as nuclear magnetic resonance (NMR), in order to fully characterize a compound for structural elucidation.
Strengths and limitations of liquid chromatography
LC is used routinely for a diverse range of applications; however, it is not suitable for the separation and analysis of volatile compounds. Robust analytical LC methods can only be realized when all the components to be separated have a lower vapor pressure than that of the mobile phase. Gas chromatography is far better suited for the analysis of volatile compounds. The nature of some biopharmaceuticals has proven challenging for LC analysis. However, advances in the equipment and techniques are helping to overcome these issues.
An extensive array of different columns and solvents are available offering a vast range in selectivity, enabling components to be separated that have a wide range of polarities. Large and small molecules are equally amenable to this technique. The ability to perform efficient separations at a relatively low temperature also makes LC an ideal separation technique for thermally labile compounds that may decompose in a gas chromatograph.
Common problems with liquid chromatography
Sample preparation is key to success; it is very important that all samples are filtered before they are loaded into the autosampler. This is especially important when working with UHPLC where high efficiency separations using sub 2-micron particles in the column are prone to blocking if samples are not filtered. The same is true for the mobile phase, especially when buffers are used.
The use of the correct injection or diluent solvent for the sample is critical, the solvent strength should be the same or less than that of the starting conditions of the mobile phase. If too strong a solvent is used, then peak splitting and poor reproducibility will be observed. A similar issue may be observed if too strong a wash solvent is used in the autosampler.
Fluctuations in the baseline of the acquired chromatogram or poor reproducibility for the retention time may well result from issues with the pump (Figure 1 (1)) or vacuum degasser. If the pump or vacuum degasser is not well maintained, a check valve may become partially stuck which will cause a pressure ripple. These issues can be resolved by ensuing that preventative maintenance tasks are carried out as per the manufacturers guidelines in order to prevent unscheduled down time and poor performance. The chromatogram can provide clues about problems with your LC.



