A Smarter Way To Remove Host Cell Protein Contamination From Gene Therapies

Complete the form below to unlock access to ALL audio articles.
What are host cell proteins and why are they important for gene therapies?
When gene therapies are created, they are manufactured using cells from cell lines. The cells function largely as biological factories to produce the proteins that constitute part of the final gene therapy product, which needs to be extracted and purified from the cells. In this process, impurities and contaminants can occur, and need to be removed. A key source of contamination is from other proteins that are produced by the host cells. These host cell proteins, or HCPs, are important because any one of them may be biologically active, even at very low concentrations. HCPs could interfere with the gene therapy's function, impacting on efficacy and safety. Even if the HCP is not bioactive in its own right, it may trigger an immune response leading to drug resistance in the patient. Therefore, HCPs are a critical quality attribute (CQA) for regulators. Detecting, monitoring and controlling HCPs is vital to ensure the quality, efficacy and safety of the gene therapy product.
This is true for all biologics. However, gene therapy products are more complex in how they are biomanufactured when compared with traditional biotherapeutics. The process introduces more sources of potentially contaminating proteins, meaning that clearing these proteins from the final product could prove more complicated and costly.
Gene therapy – it's complicated
A "typical" biologic is manufactured by expressing only one protein in a single cell line and purifying it. For a gene therapy product, viruses usually need to be incorporated into the final product. The addition of these viruses to the host cells introduces many new proteins that could co-purify with the final gene therapy product. These unwanted viral proteins also need to be detected, monitored and cleared from the final product, for the same reasons as the HCPs.
In addition, further proteins are usually added to the growth medium to help keep the cells healthy and improve their function. These proteins tend to come from a different species to the host cells. While the host cells are generally from human or other mammalian expression systems, such as from Chinese hamster ovary (CHO) cells, proteins added to the cell culture usually source intentionally from other species, such as cows for bovine serum albumin or bacteria for benzonase. Ultimately, the gene therapy developer needs to check for contaminating proteins from several very different species. Due to their speed, sensitivity, and ease of use, enzyme-linked immunosorbent assays (ELISAs) are the traditional approach in biopharma to HCP analysis. However, there is no single commercial ELISA capable of detecting such a heterogeneous mix of HCPs in a single sample.
Another difference between standard biologics and gene therapies is that when a human cell line is used, the human HCPs are not very immunogenic, resulting in ELISAs that tend to have poor coverage. This means the ELISA could fail to detect a substantial proportion of HCPs in the sample. So, commercial ELISAs alone are often not very good for detecting HCPs for gene therapy products. They simply do not have sufficient specificity for the different particular HCPs, sensitivity for HCPs in trace amounts and dynamic range for HCPs present across a broad range of concentrations.
A further complication for gene therapies versus traditional biologics is that they tend to have short development timelines. Gene therapies are mostly fast-tracked by regulators because they are aimed at treating serious illnesses, like cancer. Without suitable commercial ELISAs, drug companies would have to develop ELISAs specifically for their drug and biomanufacturing process. This typically takes 1.5–2 years, but with a compacted timeline, companies simply do not have time to develop process-specific ELISAs. Moreover, if a gene therapy is delayed getting regulatory approval because of inadequate HCP detection and clearance from the final gene therapy product, or there is insufficient data to validate the ELISA used to confirm HCP clearance, the setback could hold up drug development long enough for a competitor to bring their gene therapy to market first. As gene therapies can be one-shot cures, the first to market could effectively own the whole market.
Mass spectrometry with SWATH Acquisition meets gene therapy HCP analysis needs
To address the issues encountered with using only ELISAs for HCP detection and analysis for gene therapies, liquid chromatography tandem mass spectrometry (LC-MS/MS) is emerging as the method of choice to complement ELISA data. This is because it provides the necessary level of specificity, sensitivity and dynamic range in detecting highly heterogeneous HCPs in complex biological samples. LC-MS/MS not only offers the ability to measure thousands of proteins simultaneously, it also can quantify and characterize these proteins (see Figure 1). Further, it works for a broad range of samples, ranging from complex biologics to various gene therapies, and even new modalities such as oligonucleotide-based medicines.
 Figure 1: Using the LC-MS/MS assay with SWATH Acquisition means that all the HCPs are individually quantified and characterized. Characteristics measured include molecular weight and isoelectric point; just two of several key parameters that process developers have reported finding incredibly useful for optimizing their HCP clearance processes. The histogram above shows the molecular weight (Daltons) of each HCP identified in samples taken throughout a six-step purification process for a biologic product, analyzed using the LC-MS/MS assay with SWATH Acquisition.1
Figure 1: Using the LC-MS/MS assay with SWATH Acquisition means that all the HCPs are individually quantified and characterized. Characteristics measured include molecular weight and isoelectric point; just two of several key parameters that process developers have reported finding incredibly useful for optimizing their HCP clearance processes. The histogram above shows the molecular weight (Daltons) of each HCP identified in samples taken throughout a six-step purification process for a biologic product, analyzed using the LC-MS/MS assay with SWATH Acquisition.1
This flexibility and utility greatly address the needs of biopharma companies who are developing gene therapies. The development of a unique method and workflow for HCP detection and characterization will meet the specific needs of in various ways.1-4 The method and workflow are particularly well suited to gene therapy bioanalysis because it employs SWATH Acquisition, a data-independent acquisition technique that is applicable using TripleTOF 6600 instruments (see Figure 2). SWATH Acquisition allows for the comprehensive collection of multi-dimensional data from all the analytes in the biological sample.5,6 Such thorough, precise and robust coverage is far superior to that achievable with commercial ELISAs.1–4 By using the LC-MS/MS method, gene therapy developers can ensure that they are effectively detecting all the HCPs present in their products.
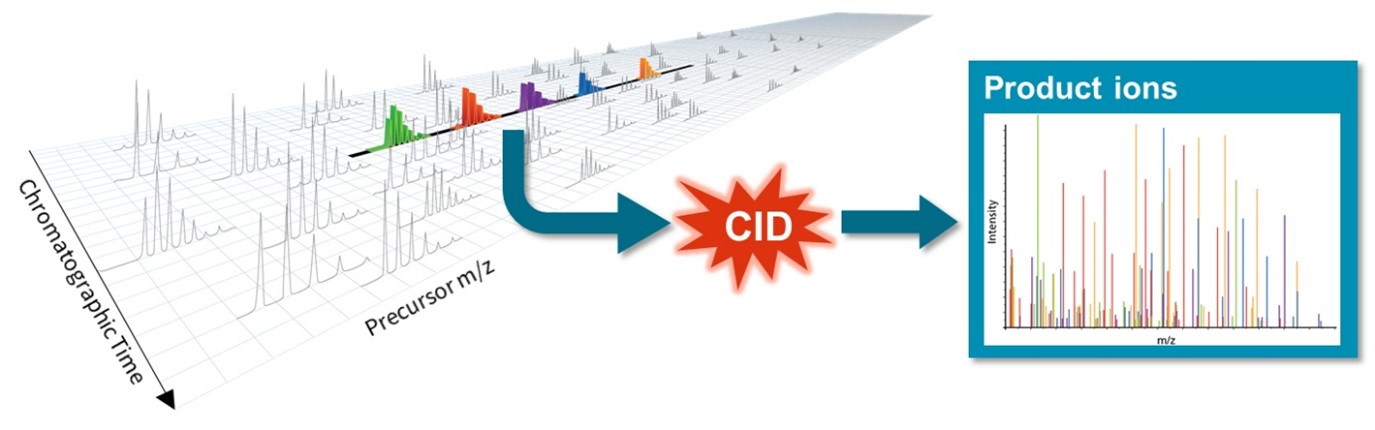
Figure 2: SWATH (sequential window acquisition of all theoretical spectra) mass spectrometry is a method for collecting the product ion spectra for all precursor ions in a sample in a time frame compatible with HPLC (high performance liquid chromatography). To accomplish this, the entire precursor ion mass range is sliced up into either automatic or user-definable ranges. Each of those ion mass ranges are sent as a single packet for CID (collision induced dissociation) fragmentation. The subsequent composite product ion spectrum is then recorded. The process is repeated at very high speed for every other mass range. This same process repeats for every single measurement cycle. It yields a comprehensive and quantifiable measurement of every precursor and every product ion in a sample.5
LC-MS/MS is critical in many ways for gene therapy development
The data provided by LC-MS/MS is richer than that obtained with ELISAs. While ELISAs provide a number that is rather arbitrary, LC-MS/MS provides absolute quantification of each HCP. Moreover, ELISAs often produce different results from batch to batch and as such are not reproducible. This is extremely frustrating for gene therapy developers.
In contrast, LC-MS/MS is highly reproducible. LC-MS/MS can be used in a number of ways:
- To inform process development
- To inform and validate the choice of commercial ELISAs
- To even replace ELISAs entirely
While ELISAs are still favoured for cost, convenience and current regulatory compliance, LC-MS/MS assays can help determine which commercial ELISA is best for a particular project – also providing the evidence to validate its selection. LC-MS/MS is also a powerful process-development tool, as it has comprehensive coverage and measures the physicochemical properties of each analyte. Armed with this information, developers can make simple tweaks to their filters and/or buffers to completely eliminate the HCPs.
MS data for ELISA validation or direct profiling of HCPs is becoming an increasingly important factor for regulatory authorities. Using SWATH Acquisition means that the data collected is effectively immortalized, which may be more meaningful as libraries and databases become increasingly well annotated with time. Many large biopharma companies predict that it is simply a matter of time before regulators require mandatory MS data on their HCP analysis, which is why they have invested in pre-emptively including LC-MS/MS into their HCP analysis and risk management strategy. Further risk is minimized as the LC-MS/MS provides confidence and security regarding any ELISA or other HCP analysis performed, ensuring that all HCPs have been fully accounted for throughout the entire drug development lifecycle.
Working together to bring novel gene therapies to clinics faster, safer and cheaper
In comparison with the usual 1.5–2 years it takes to develop a process-specific ELISA, LC-MS/MS assays generally takes only six-to-eight weeks to optimize and establish for any biologic product, from project start to the receipt of a regulatory verification notice. Thus, LC-MS/MS can greatly accelerate the development, marketing approval and commercial biomanufacture of novel gene therapies.
Dr Ejvind Mørtz, PhD, is co-founder and COO of Alphalyse. He has more than 20 years of experience from several biotech companies. His experience includes the development of protein analysis methods in research and CMC development of protein biologics, and business collaborations with CMOs/CROs. He has a Business Executive education from Harvard Business School. As COO of Alphalyse, he drives the development and improvement of analytical services for customers.
Todd Stawicki is the Global Marketing Manager for BioPharma LC-MS at SCIEX. He has over 15 years of experience as a research scientist at several pharmaceutical and biopharmaceutical companies and as an LC-MS applications scientist at SCIEX. In his role as a marketing manager, he is passionate about empowering customers with the most innovative and effective LC-MS methods available to achieve their scientific goals.
References:
1. Lund RR, Pilely K, Mørtz E. Using LC-MS for Efficient HCP Clearance. BioPharm International. June 30, 2019. http://www.biopharminternational.com/using-lc-ms-efficient-hcp-clearance (accessed April 2020).
2. Lund RR, Nielsen SB, Crawford J, et al. Identification and Absolute Quantification of Individual Host Cell Proteins by SWATH® LC-MS. https://www.alphalyse.com/wp-content/uploads/2018/11/poster_hcp_id_and_quantification_by_swath.pdf (accessed April 2020).
3. Heissel S, Neffling M, Lund RR, et al. Host Cell Protein Analysis by Microflow-LC High Resolution SWATH-MS of Vaccine Samples Under Development. The American Society for Mass Spectrometry (ASMS) Annual Conference 2016. Poster ID: WP 628. https://sciex.com/Documents/posters/asms2016_628_Wed_Heissel_Neffling.pdf (accessed April 2020).
4. Carapito C. Dual Data-Independent Acquisition Method using SWATH® Acquisition-MS for Host Cell Proteins (HCP) Profiling and Absolute Quantification of Key Impurities during Bioprocess Development. On-demand Webinar. SCIEX. https://sciex.com/events/hcp-profiling-quantification-impurities (accessed April 2020).
5. SCIEX. SWATH Acquisition, Ensuring Nothing is Missed. https://sciex.com/technology/swath-acquisition (accessed April 2020).
6. Ivosev G, Cox DM, Bloomfield N, et al. Scanning SWATH® Acquisition Method for Improved Compound Screening. https://sciex.com/Documents/posters/asms2018/Technology/354_Monday_Ivosev.pdf (accessed April 2020).

