Chemists Develop Method to Synthesize Drugs From Renewable Precursors

Complete the form below to unlock access to ALL audio articles.
The scientists of RUDN University together with their Russian colleagues have developed a new approach to the synthesis of benzofurans from cheap raw materials. Original furans can be produced from wastes of agriculture and wooworking industry, such as sawdust, cobs and other by-products of crop production. The results of the work were described in the article published in Tetrahedron.
Benzofuran is a heterocyclic compound consisting of fused benzene and furan rings. The last one is five-membered ring formed by four carbon atoms and an oxygen atom. Benzofuran fragment is present in many pharmaceutical substances such as antiarrhythmic medications (Amiodarone and Dronedarone), medications for psoriasis and gout (Benzbromarone and Benziodarone), drugs for the treatment of sleep disorders (Heltioz) and skin diseases (Psoralen and Trioxalen) as well as some antidepressants. It is also important that the new approach can be used for the development of new benzofuran-based drug substances.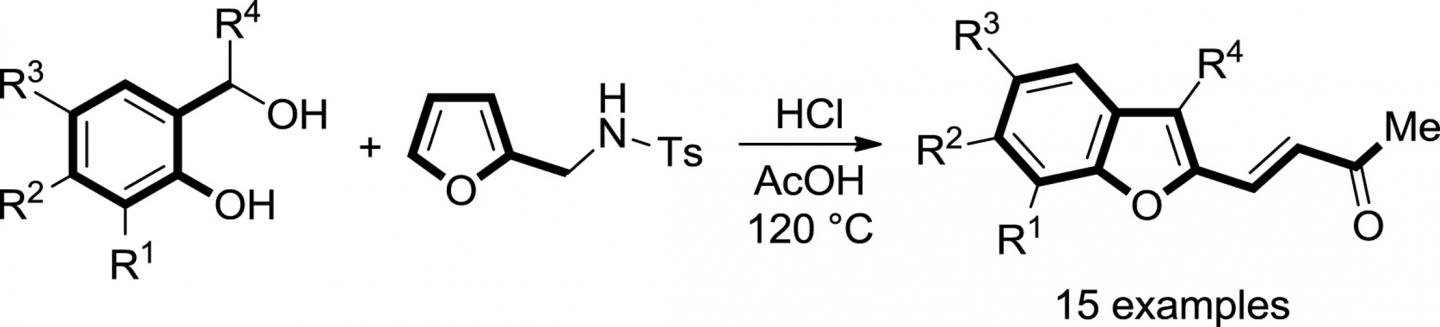
This is the novel simple synthetic pathway to obtain benzofurans from salicyl alcohols and N-tosylfurfurylamine. Credit: Igor Trushkov
"Original furans (N-tosylfurfurylamines) are obtained directly from agricultural or wood-processing waste, that is, from renewable sources. Biomass processing will eventually come to a serious industrial level, so there will only be more precursors for synthesis we are engaged in", one of authors of the work, Professor of the Department of Organic Chemistry at RUDN University Igor Trushkov commented, who is also the head of the laboratory of chemical synthesis at Dmitry Rogachev National Research Center of Pediatric Hematology, Oncology and Immunology.
The second component necessary for the proposed synthesis is salicyl alcohols, which are also produced commercially from a cheap precursor, that is salicylaldehyde which is obtained in one step from phenol, large-tonnage product of chemical industry.
"This work is also interesting because in the reaction with salicylic alcohols the same atom of the furan ring acts at first as a nucleophile (a reagent providing an electron pair for the formation of new bond), and then, during the same process, as an electrophile (a reagent which accepts an electron pair)", Igor Trushkov adds, "Shampoo and conditioner together in one bottle, so to speak. Or rather (given that shampoo and conditioner are almost the same thing, while an electrophile and a nucleophile are exactly opposite) union of angel and demon".
In close cooperation with a group of scientists from Perm State National Research University, led by Maxim Uchuskin, for many years Igor Trushkov has been searching for ways to synthesize various heterocyclic compounds from low-cost precursors. The beginning of this field was made by Professor Alexander Butin, after whom one of the reactions was named.
This article has been republished from materials provided by RUDN University. Note: material may have been edited for length and content. For further information, please contact the cited source.
Reference
Merkushev, A. A., Strelnikov, V. N., Uchuskin, M. G., & Trushkov, I. V. (2017). A simple synthesis of benzofurans by acid-catalyzed domino reaction of salicyl alcohols with N -tosylfurfurylamine. Tetrahedron, 73(46), 6523-6529. doi:10.1016/j.tet.2017.09.043

