A “Losing” Proposition: Tackling the Problem of Lost or Unrecovered Analytes in Chromatography

Complete the form below to unlock access to ALL audio articles.
Chromatography, and in particular high-performance liquid chromatography (HPLC), has proven over the years to be an invaluable tool in analytical labs. From food analysis, to environmental testing and pharmaceutical safety, HPLC plays a critical role. However, despite being a “mature” technique, there are still some areas that prove problematic, leading to suboptimal results.
One such area is the loss of analytes during the chromatographic process. This stems from interactions that occur between the analytes in the sample and the metal surfaces present in the equipment. We spoke to Amit Patel, Kim Haynes, Kerri Smith and Martin Gilar, Waters Corporation, about the challenges of analyte loss during HPLC, the problems it causes and how innovations in this space are helping to overcome the issues.
Q: Analyte loss due to binding and adsorption of analytes during chromatographic separations has been a problem since the beginning of HPLC separations. What is the impact of this problem on analytical results?
A: Since the advent of HPLC, we have seen many advances in technologies and methodological strategies for mitigating the chromatographic effects of surface metal adsorption; a common topic that has been the subject of many product catalogs and journal articles and a focus of routine laboratory practices. Non-specific analyte binding and adsorption leads to the deterioration of peak shape and the loss of peak area in the best of circumstances, while poor assay-to-assay precision or total peak loss occur in the worst of circumstances. Furthermore, the extent of metal-binding differs across chromatographic technologies and is magnified by variably effective masking practices that hinder the interpretation of results.
Q: What has Waters done to address this challenge?
A: Waters has developed a group of technologies, named MaxPeak™ High Performance Surfaces (HPS), with the goal of mitigating sample losses due to analyte/surface interactions. One of these is a hybrid organic-inorganic surface chemistry to help address the challenge of analyte-metal surface interactions in liquid chromatography. This technology is based on an ethylene-bridged siloxane chemistry. It forms a barrier between the sample and metal surfaces of both the system and column and minimizes metal-based adsorption, improves reproducibility, simplifies methods development and enhances sensitivity and peak shape. It also eliminates the need for surface passivation which is the process of removing free metal ions by means such as acid treatment. The new chromatographic columns and systems from Waters with MaxPeak HPS technology all carry the PREMIER brand name.
Q: What is the difference or advantage of the PREMIER solution, versus other solutions?
A: The advantages of the Waters™ ACQUITY PREMIER™ solution depend on what alternatives we compare against. The use of chelating agents with mobile phases is one example. Other examples include surface conditioning with repeated sample injections to occupy active sites on metal surfaces and sample derivatization to alter analytes to circumvent electrostatic interactions. Each of these work-around procedures unnecessarily complicates analyses by introducing additional steps into a workflow. These workarounds provide temporary benefits, introduce the potential for contamination and in some cases cause ion suppression for LC-MS analyses.
Other than methodological alternatives to counteract non-specific adsorption, some solutions for this challenge include utilizing alternative materials for the surfaces of chromatographic columns and system components. One of these materials is polyether ether ketone (PEEK). While effective for reducing metal-based interactions, PEEK is limited to lower operating pressures and is subject to swelling by mobile phase solvents like dimethyl sulfoxide (DMSO) and tetrahydrofuran (THF).
With the Waters ACQUITY PREMIER Solution, it is possible to get sharp peaks and greater peak capacity from the very first injection without complicating the analysis with mobile phase additives like chelating agents or with time-consuming passivation and surface conditioning.
Q: Can you provide a few examples of the performance improvements?
A: The ACQUITY PREMIER Solution is designed to alleviate the problem of analyte/metal surface interactions when analyzing organic acids, organophosphates, oligonucleotides, phosphopeptides, acidic glycans and phospholipids by reversed-phase and hydrophilic interaction chromatography.
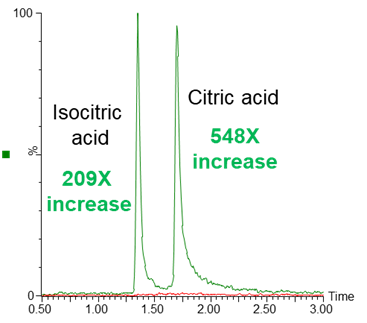
The use of an ACQUITY PREMIER CSH Phenyl-Hexyl column compared to a conventional CSH Phenyl-Hexyl column for the analysis of citric and isocitric acid yielded substantially greater peak area under equivalent analysis conditions (Figure 1). These tricarboxylic acid (TCA) cycle analytes contain carboxylate moieties which are known to bind to metal. Profound chromatographic performance differences are observed when these interactions are prevented with the use of an ACQUITY PREMIER column.
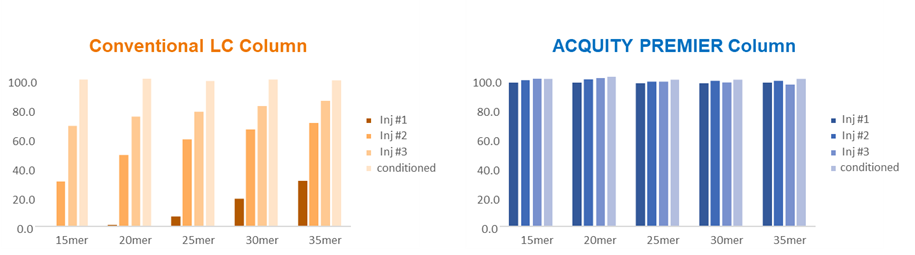
Figure 2: Bar plots showing performance of conventional and ACQUITY PREMIER columns with values for the first three sample injections as well as post conditioning with 500 pmol of 15-35 mer oligodeoxythymidine standard, followed by a 10 pmol standard injection. Sample injections were 2 µL of 10 pmol of the oligonucleotide standard injected on column. The columns used in these measurements were the standard and ACQUITY PREMIER versions of BEH C18 Oligonucleotide (2.1 x 50 mm, 1.7 mm). Credit: Waters Corporation.
Yet another benefit of the MaxPeak HPS technology is the reduced need for surface conditioning. Practitioners often perform multiple injections of the sample as a conditioning step prior to the analysis of samples that are susceptible to adsorption to metal surfaces. As shown in Figure 2, a conventional column requires multiple injections to saturate the active adsorption sites and achieve consistent sample signal. Oligonucleotides belong to a class of analytes for which surface adsorption can affect recovery and peak area counts. Here we demonstrate the surface conditioning is not required to achieve repeatable signal when using an ACQUITY PREMIER column instead of a conventional column.
Q: What do you think the future looks like for an expansion of this technology?
A: Liquid chromatography instruments and columns are made substantially of metal which negatively affects peak shape, analyte recovery and reproducibility. The prevalence and severity of undesired binding and adsorption during chemical measurement are undeniable and highlight the value that MaxPeak HPS brings to analyte recovery, assay-to-assay reproducibility, and to separation scientists with a greater level of assurance in the integrity of their qualitative and quantitative analytical results.
Dr Amit Patel, Senior Scientist, Kim Haynes, Principal Product Marketing Manager, Dr Kerri Smith, Principle Scientist and Dr Martin Gilar, Principle Scientist, all Waters Corporation, were speaking to Dr Karen Steward, Senior Science Writer for Technology Networks.


