Development of Immunotherapy To Treat COVID-19 in Progress
Complete the form below to unlock access to ALL audio articles.
CEL-SCI Corporation recently announced that it is utilizing LEAPS peptide technology in efforts to develop an immunotherapy against the novel coronavirus caused by SARS-CoV-2.
The technology utilizes conserved regions of the SARS-CoV-2 protein to stimulate a protective T cell response, reducing viral load. Furthermore, LEAPS can also be adopted to create peptides with antiviral and anti-inflammatory characteristics for immunotherapeutic applications. Preclinical studies have demonstrated that LEAPS immunogens are able to prevent infection by herpes simplex virus (HSV) and influenza A in animal models.
Technology Networks spoke with Daniel Zimmerman, Ph.D. Senior VP of Research, Cellular Immunology at CEL-SCI Corporation, to learn more about the LEAPS technology and its potential application in treating COVID-19.
Molly Campbell (MC): For our readers that are unfamiliar, please can you describe what immunotherapy entails?
Daniel Zimmerman (DZ): Immunotherapy entails the administration of agents (e.g., cellular alone or better still complexes combined with another immune agent or better still with either proteins, better antibodies alone or even better still specific peptides, etc.) that activate the immune system or act to induce a desired endogenous immune response in the recipient.
Laura Lansdowne (LL): What parallels can be drawn between coronavirus and the other respiratory viruses you have previously dealt with?
DZ: The novel COVID-19 a very close cousin to SARS, and MERS is e like influenza A in that all these infect the lung and initiate tissue damaging inflammatory responses. The LEAPS (Ligand Epitope Antigen Presentation System) technology was previously used to develop immunotherapy for an influenza A (H1N1) in collaboration with the NIAID-NIH. In, these viruses target both the upper respiratory system and the lungs.
Virus replication and inflammatory responses result in the disease-causing tissue damage. Individuals whose immune system cannot defend against the virus quickly, or who overly respond to the virus with inflammation, are at highest risk of serious disease consequences, in addition to other “at risk” groups including elderly populations.
LL: Can you elaborate on how the LEAPS peptide technology could be a tool against the COVID-19 coronavirus?
DZ: Immunotherapy can be either antigen-specific or non-specific. In the case of the LEAPS Technology for the COVID-19 coronavirus, we envision an antigen-specific antigen disease specific immune therapy that uses hetero-conjugate of peptides designed to target specific viral epitopes while focusing on stimulation of the immune cells that drive the proper response.
Specific classes or sub-classes of T cells within the CD4 or CD8 categories will be targeted. These cells stimulate and may recruit other immune cells, leading to highly significant reduced virus levels and beneficial changes in cytokine (immunological hormones) profiles in the lung tissues of patients.
They either cells or cytokines may also cause lysis of virally infected cells to decrease the virus load affecting the body. It is equally important to elicit protective immune cellular responses while minimizing tissue damaging inflammation.
Our studies will build on previous immunotherapeutics that were developed against influenza A subtype H1N1 in collaboration with the NIAID-NIH. In this work, LEAPS peptides H1N1 peptides halted the progression of disease, reducing viral presence in the lung tissues and inducing cytokine profiles that provided a balance of pro-inflammatory and anti-inflammatory regulatory responses.
In summary, LEAPS immunotherapy can be designed to stimulate the appropriate immune cells to deal with the virus infection in a balanced manner. We will be able to observe this by monitoring prevention or mitigation of viral disease and the nature of the cytokines that are present in the lung or blood after administration.
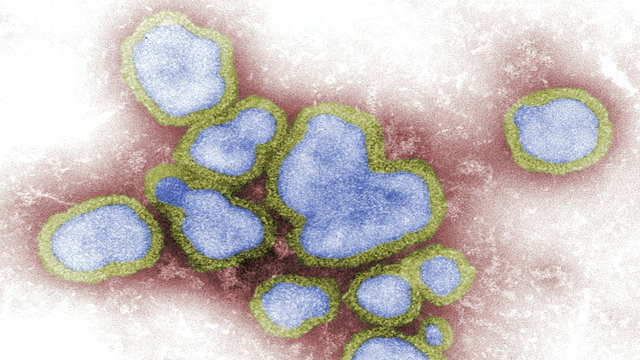
This digitally-colorized, negative-stained transmission electron microscopic (TEM) image depicts a number of Influenza A virions. Credit: Unsplash.
MC: The previous studies in collaboration with NIAID showed LEAPS peptides reduced morbidity and mortality in mice with pandemic influenza. As these are animal studies, how translational do you think the findings are to humans?
DZ: The influenza studies built on findings that arose from studies of LEAPS immunotherapies for herpes simplex virus (HSV). In these studies, the LEAPS peptides elicited equivalent immune responses in human and mouse cells. In addition, as for the influenza studies, the antigenic peptides to be selected for the LEAPS immunotherapy will be from highly conserved sequences (i.e., do not change in the virus) that are recognized by the immune systems of both humans and mice.
MC: What are the key challenges in developing an effective treatment for COVID-19?
DZ: The first challenge in developing any immunotherapy is to select the peptides that contain the proper antigenic epitope sequences. These sequences need to be recognizable by the immune system in addition to the LEAPS immune cell binding ligand (ICBL) to drive the appropriate response. This has already been achieved.
Viral peptide sequences were chosen based on protein sequences that are very similar to MERS and SARS coronaviruses. The ICBL was chosen based on experience with HSV and influenza, TB, HIV and other studies, but another ICBL will also be tested as a control and to hedge our bets and expedite the development process.
MC: What are your key priorities in terms of taking a LEAPS/COVID-19 product forward?
DZ: The first priority is to demonstrate that appropriate immune responses are elicited against the LEAPS immunotherapies in animal studies and then to demonstrate protection from lethal infection of Covid-19. Discussions have been initiated with several agencies and clinical research organizations (CROs) to develop studies designed to determine the potential of LEAPS immunotherapy against COVID-19 and for producing GMP grade materials for clinical studies after GLP safety studies in animals.
Daniel Zimmerman, Ph.D. Senior VP of Research, Cellular Immunology at CEL-SCI Corporation, was speaking to Molly Campbell and Laura Elizabeth Lansdowne, Science Writers, Technology Networks.




