Democratizing NIR Spectroscopy

Want to listen to this article for FREE?
Complete the form below to unlock access to ALL audio articles.
Read time: 2 minutes
Process Analytical Technology tools play a vital role in QbD, but are often both difficult and expensive to install. Pharmaceutical manufacturers also increasingly require tools which enable in-line monitoring, as they move towards continuous manufacturing.
We spoke to Nada O'Brien, Director, Marketing & Product Management at Viavi, to learn about the improvements that Viavi’s recently launched MicroNIRs can bring to pharmaceutical manufacturers.
AM: What are some of the challenges that pharmaceutical drug manufacturers face when implementing Quality-by-Design operations?
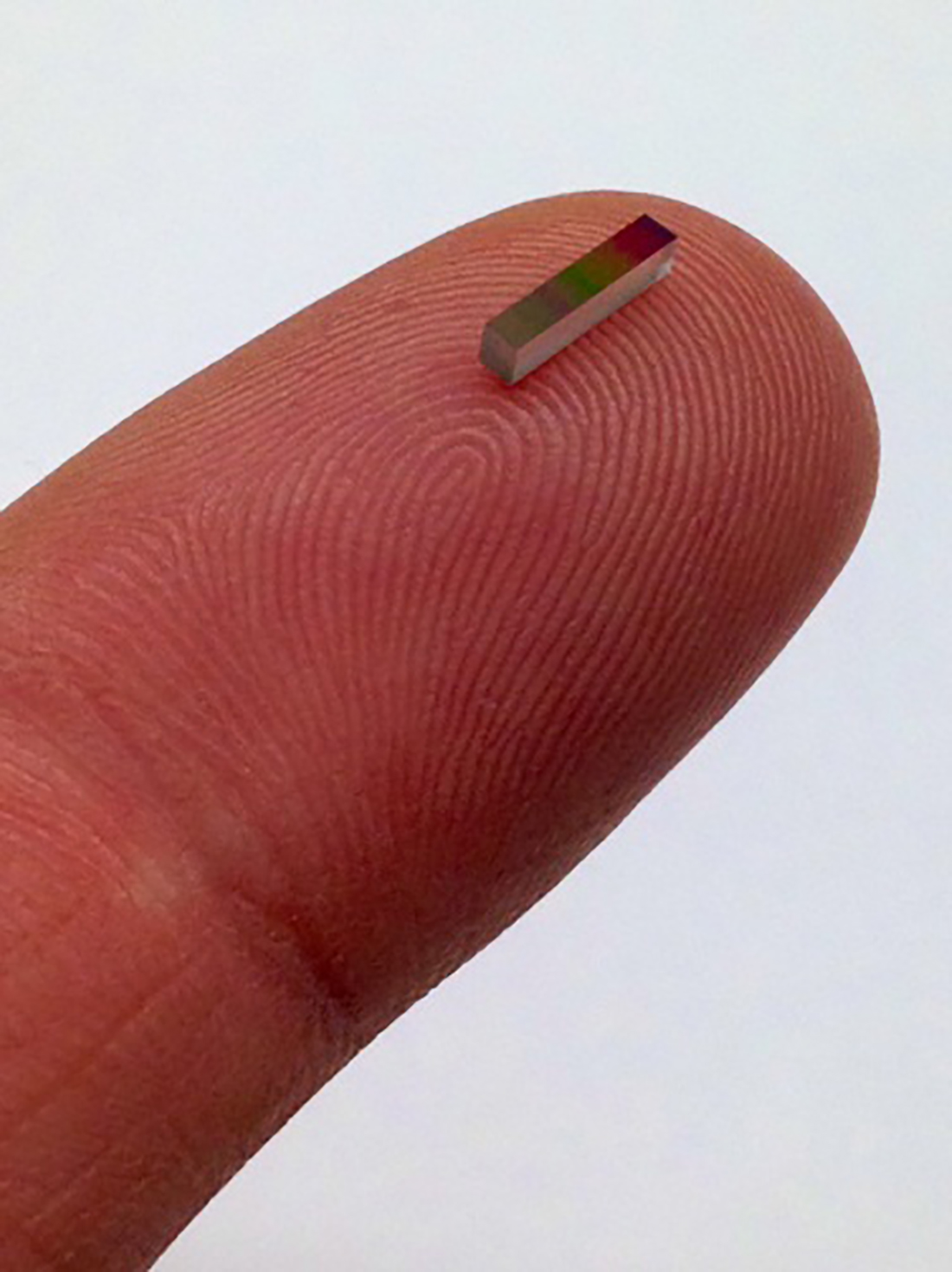
NO: An essential part of implementing QbD is the use of Process Analytical Technology (PAT) tools, such as NIR spectroscopy or particle size analyzers that monitor aspects of the process (e.g., blending, drying, granulation, tableting) and provide real-time data on staying within the required upper and lower process control limits. PAT tools like NIR spectroscopy have traditionally been bulky, requiring not only high capital expenditures, but also requiring complex installations on existing unit operations. With the small form-factor, cost-effectiveness and ease of adapting (and installing) MicroNIR on production lines, the vision is MicroNIR now facilitates a much greater adoption of such tools as ubiquitously and almost as easily as installing temperature or flow sensors. MicroNIR is democratizing the NIR spectroscopy for use everywhere, anywhere where organic materials are to be monitored. This is particularly true in solid dose manufacturing of pharmaceutical drugs.
AM: Can you describe some of the limitations of current post-production monitoring and analysis methods?
NO: Post-production analysis of samples means taking a thief sample from a given batch process, after it has been completed, to the lab for analysis. The material produced from that process will usually remain under quarantine until the tests results are back from the lab. This wait time which could be up to a full day or longer costs money. The machine is idled until the material is deemed ‘pass’ or ‘fail ‘ and before it is to be moved on to the next step. If the batch is found ‘out of specification’, the material has to be disposed of. However, when applying in-line monitoring, the process engineer or process owner is able to be notified of an out-of-spec condition immediately before a batch of production is complete. S/He can react in real-time to course-correct the situation before a batch of bad material is produced and wasted.
Furthermore, the new trend of moving from batch processes to continuous solid dose manufacturing processes means the process has to be monitored and controlled by in-line. Instruments like MicroNIR are a must. Thief samples and taking samples to the lab are not an option in continuous manufacturing.

Nada O'Brien was speaking to Anna MacDonald, Editor for Technology Networks.
We spoke to Nada O'Brien, Director, Marketing & Product Management at Viavi, to learn about the improvements that Viavi’s recently launched MicroNIRs can bring to pharmaceutical manufacturers.
AM: What are some of the challenges that pharmaceutical drug manufacturers face when implementing Quality-by-Design operations?
NO: An essential part of implementing QbD is the use of Process Analytical Technology (PAT) tools, such as NIR spectroscopy or particle size analyzers that monitor aspects of the process (e.g., blending, drying, granulation, tableting) and provide real-time data on staying within the required upper and lower process control limits. PAT tools like NIR spectroscopy have traditionally been bulky, requiring not only high capital expenditures, but also requiring complex installations on existing unit operations. With the small form-factor, cost-effectiveness and ease of adapting (and installing) MicroNIR on production lines, the vision is MicroNIR now facilitates a much greater adoption of such tools as ubiquitously and almost as easily as installing temperature or flow sensors. MicroNIR is democratizing the NIR spectroscopy for use everywhere, anywhere where organic materials are to be monitored. This is particularly true in solid dose manufacturing of pharmaceutical drugs.
AM: Can you describe some of the limitations of current post-production monitoring and analysis methods?
NO: Post-production analysis of samples means taking a thief sample from a given batch process, after it has been completed, to the lab for analysis. The material produced from that process will usually remain under quarantine until the tests results are back from the lab. This wait time which could be up to a full day or longer costs money. The machine is idled until the material is deemed ‘pass’ or ‘fail ‘ and before it is to be moved on to the next step. If the batch is found ‘out of specification’, the material has to be disposed of. However, when applying in-line monitoring, the process engineer or process owner is able to be notified of an out-of-spec condition immediately before a batch of production is complete. S/He can react in real-time to course-correct the situation before a batch of bad material is produced and wasted.
Furthermore, the new trend of moving from batch processes to continuous solid dose manufacturing processes means the process has to be monitored and controlled by in-line. Instruments like MicroNIR are a must. Thief samples and taking samples to the lab are not an option in continuous manufacturing.
AM: What improvements can Viavi’s MicroNIRs bring to pharmaceutical manufacturers?
• The ability to provide a cost effective process monitoring solution that enables smarter ways of doing manufacturing.
• Ease of adoption and installation on process equipment
• Ability to use data not only to determine the process end-point, but to also help understand how the process behaves that allows process engineers do continuous improvements.
• Ability to install several MicroNIR sensors on a given process and not just one location as a way to understand process/reaction dynamics. Similarly, the ability to monitor multiple sensors from several different similar processes in a global operation to better understand differences from one site to another. This is at the heart of the 4th industrial revolution or the industrial internet of things.
• Essential for not only monitoring but control of a process in a continuous manufacturing process.
AM: Can you tell us more about the Linear Variable Filter technology that powers the MicroNIR spectrometers?
• The ability to provide a cost effective process monitoring solution that enables smarter ways of doing manufacturing.
• Ease of adoption and installation on process equipment
• Ability to use data not only to determine the process end-point, but to also help understand how the process behaves that allows process engineers do continuous improvements.
• Ability to install several MicroNIR sensors on a given process and not just one location as a way to understand process/reaction dynamics. Similarly, the ability to monitor multiple sensors from several different similar processes in a global operation to better understand differences from one site to another. This is at the heart of the 4th industrial revolution or the industrial internet of things.
• Essential for not only monitoring but control of a process in a continuous manufacturing process.
AM: Can you tell us more about the Linear Variable Filter technology that powers the MicroNIR spectrometers?

NO: LVF is a space-qualified component. It is comprised of high reliability thin film optical coatings. The LVF replaces the diffraction grating that is typically found in bench top or other small optical NIR spectrometers. Since it’s a coating on glass, the LVF is mounted directly over a detector array. This reduces the size significantly and provides good thermal and optical stability.

Nada O'Brien was speaking to Anna MacDonald, Editor for Technology Networks.

