Chronic Skin Inflammation Molecular Mechanisms Identified
Complete the form below to unlock access to ALL audio articles.
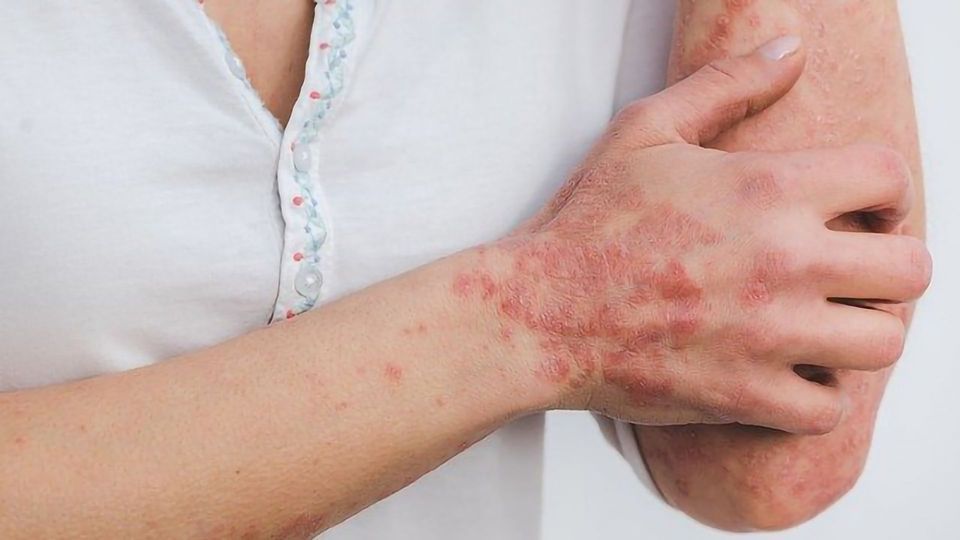
Frequently occurring chronic skin inflammation like in atopic dermatitis (AD or neurodermatitis) and psoriasis have different causes such as genetic predisposition, stress or allergens. These frequently occurring skin diseases are mostly attributed by biomedical scientists to a disturbed immune system, although the noticeable thickening and flaking of the epidermis, which is the outermost layer of skin, also indicates a disruption of the epithelial cells. A team of researchers from the University Clinic for Dermatology and the Clinical Institute for Laboratory Medicine at MedUni Vienna has now been able to identify new molecular mechanisms as causes that could provide suitable starting points for new therapies.
Using patient samples and animal models, the researchers were able to show that a multifunctional protein called "p62" influences the inflammatory changes in diseased epidermis and that inhibiting p62 leads to an alleviation of chronic inflammation. Sequestosome 1 / p62 is a multifunctional protein that affects the control of signal transduction and cellular balance ("homeostasis)" explains Erwin Wagner, head of the study from the University Clinic for Dermatology and the Clinical Institute for Laboratory Medicine at MedUni Vienna.
The present study therefore examined whether p62 plays a role in the development of atopic dermatitis (AD). Wagner: "For this purpose, AD-like skin lesions were induced by genetic inactivation of a certain gene, called JunB, in keratinocytes - this is the type of cell mainly found in the epidermis - which led to an increase in the expression of p62 in the skin of mice." The contribution of p62 to pathological changes was then determined by the additional genetic inactivation of p62.
New therapy option for AD and related skin diseases
The result: The loss of p62 reduced skin damage, suggesting that the inhibition of p62-dependent signals could improve the clinical picture of atopic dermatitis (AD) and possibly also related skin diseases such as psoriasis. The researchers were also able to detect increased amounts of p62 in skin sections from patients with AD and psoriasis. Further investigations showed that the inactivation of p62 normalized the altered differentiation of epidermal keratinocytes, reduced the thickening of the epidermis and decreased the infiltration of immune cells.
"Both the visible skin lesions were significantly reduced, as was the circulating immunoglobulin E (IgE) in the blood," says Wagner, summarizing the results. High IgE levels are a typical characteristic of AD patients. At the molecular level, in turn, p62 activates certain signalling pathways that play a major role in inflammatory processes. In the absence of p62 or by therapeutic blockade, these signalling pathways are not activated, which underlines the important role of p62 in AD-like inflammation.
Wagner: "These results provide the first in vivo evidence for an inflammatory role of p62 in the skin and suggest that p62-dependent signalling pathways are promising therapeutic targets for ameliorating the skin manifestations of AD and possibly also psoriasis."
Reference
Sukseree S, Bakiri L, Irigoyen MP, Uluçkan Ö, Petzelbauer P, Wagner EF. Sequestosome 1/p62 enhances chronic skin inflammation, J Allergy Clin Immunol, (2021). doi:https://doi.org/10.1016/j.jaci.2021.02.028
This article has been republished from the following materials. Note: material may have been edited for length and content. For further information, please contact the cited source.

