Optimizing Optical Technology for Automated Hematology Analyzers

Complete the form below to unlock access to ALL audio articles.
Automation of cell counting and characterization has revolutionized the results of hematology analysis since its initial development in the 1960s. Although many detection methods are still in use, optical technology has represented a key innovation in automated hematology analysis since its introduction.1,2,3,4
Light, scattered and detected in specific angles, captures an array of information about cell size, structure, inner complexity, nuclear segmentation and cytoplasmic granulation. In particular, innovative expansions of optical flow cytometry have enabled laboratories to obtain higher quality results, for more accurate and rapid clinical decision making.
The most common technologies are electrical impedance, radio frequency (RF) conductivity, optical light scatter (optical flow cytometry), cytochemistry and fluorescence. Optimal combinations of these detection methods provide an accurate automated complete blood count (CBC), including white blood cell (WBC) differential, in a short turnaround time.
The evolution of WBC differentials
The automated WBC differential provides the absolute and relative (%) concentrations of the five different types of WBCs (neutrophil, eosinophil and basophil granulocytes, lymphocytes and monocytes) present in normal blood. This provides information for diagnosis and assessment of infections, immune system or bone marrow disorders, and hemato-oncological diseases. Traditionally, the WBC differential has been determined by manual counting and classification of 100 or 200 WBCs on a stained blood smear.5 This method, although highly imprecise6, is still considered the reference method for the WBC differential7, and may be performed as a reflex test after an automated CBC analysis.Today, WBC differentials are usually performed on automated hematology analyzers. Although the automated WBC differential is highly accurate and reliable in the absence of pathological cell types, a manual differential is often required to confirm the presence of immature or reactive cells, blasts, and other pathological cell types. Figure 1 shows the development of WBC differential technology since its initiation in the 1950s.
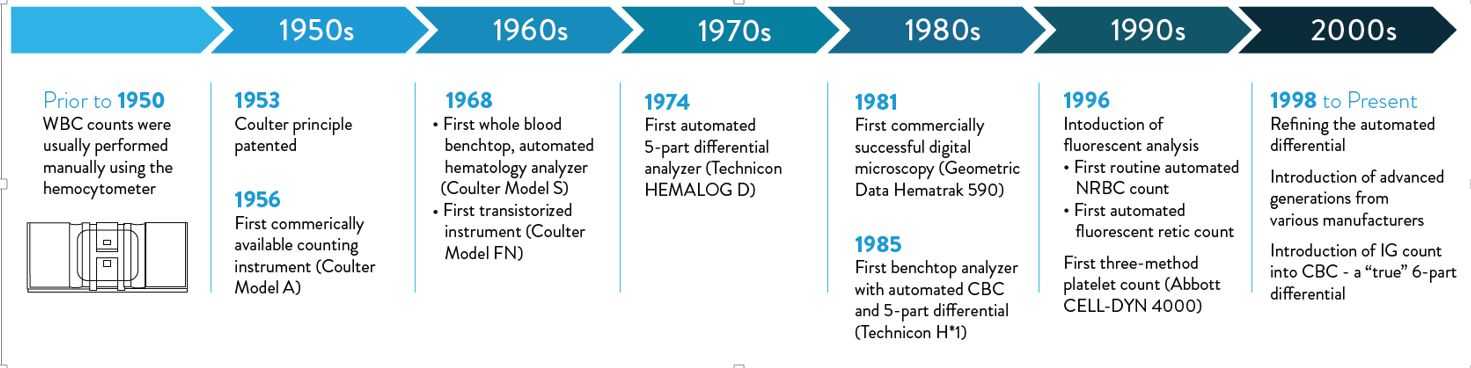
Figure 1: Automated white blood cell (WBC) differential count technology development timeline.
Impedance and cytochemistry
Electrical impedance is a cell counting and sizing technique based on the measurement of changes in electrical impedance (resistance) produced by a particle (i.e. a blood cell). It was the first automated technology to count cells and measure the size of WBCs, red blood cells (RBCs) and platelets (PLTs). As cells pass through an aperture of known size, they cause a change in electrical conductance which is proportional to the size of the particle (Coulter Principle8, Figure 2a).Impedance technology can deliver a three-part WBC differential, where cells are grouped into three sizes: lymphocytes, mid-range cells and granulocytes (Figure 2b), but it does not allow for the differentiation of the granulocyte sub-type. The limitation of this technology is that it is based on purely measuring cell size, so abnormal cells, such as nucleated red blood cells (NRBCs), PLT clumps, giant PLTs or un-lysed red cells, cannot be separated and may interfere with normal cell populations.
Some current hematology analyzers still rely on impedance technology, although with additional technologies to improve the differential white cell count. The utilization of a high frequency signal, defined as RF conductivity, allows the discrimination between different WBC subpopulations.8 RF signals pass through the cell, producing a response that is related to nuclear composition, cytoplasmic density and other differences in internal structures. This technology aims at mitigating the inherent limitations of impedance technology.
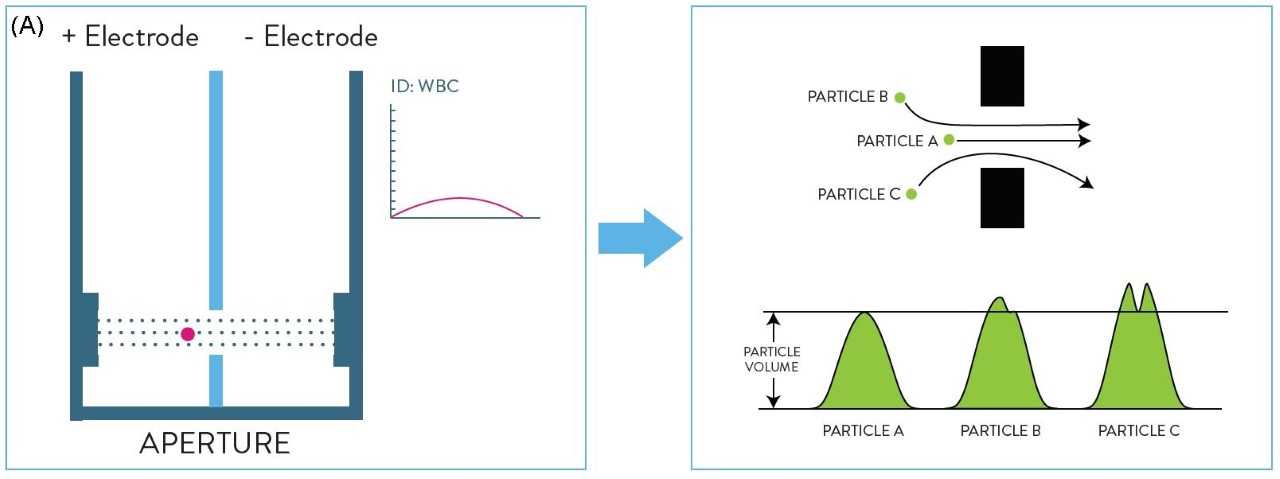
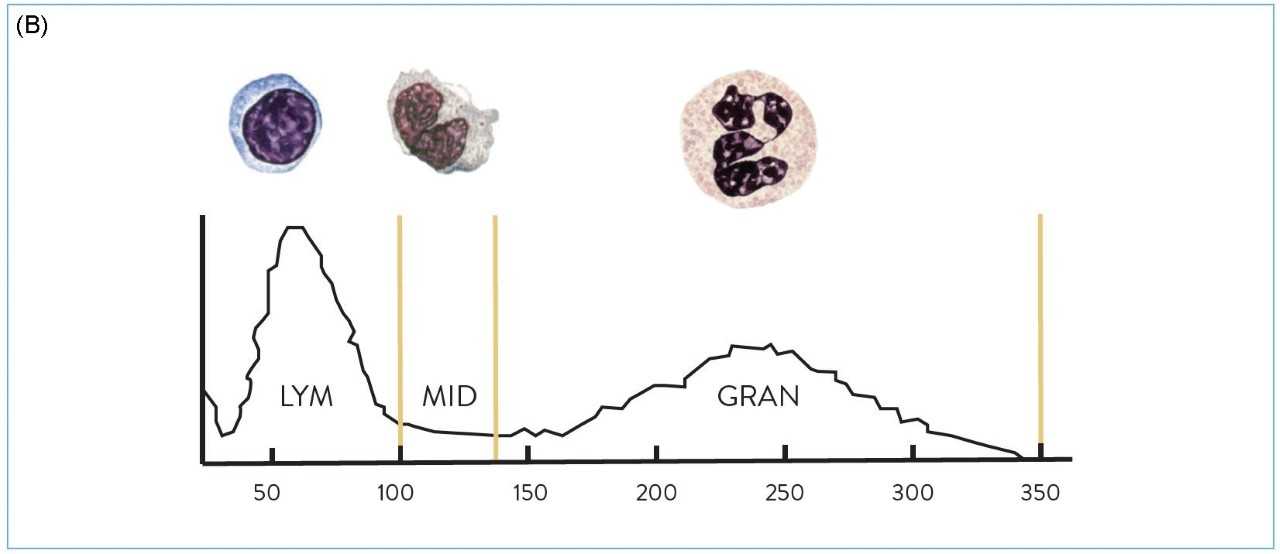
Figure 2: (A) Representation of the Coulter Principle (electrical impedance). (B) Three-part white blood cell (WBC) differential by impedance.
Cytochemical staining is another technology used to distinguish between cells containing myeloperoxidase (a lysosomal enzyme located in the azurophilic granules of the neutrophils and its precursors, eosinophils and monocytes) and peroxidase-negative cells, which include lymphocytes and basophils.9
Optical technologies
Optical flow cytometry provides several advantages over traditional impedance methods. During optical light scatter measurement, a beam of laser light is passed through a diluted blood specimen stream that is projected into the flow cell by hydrodynamic focusing. As each cell passes through, the focused light is scattered in various directions and detected by photodetectors which convert the signal into an electric pulse. The electronic signals are transmitted to a computer for storage and analysis.The signals provide information about cellular characteristics such as size, internal complexity, nuclear lobularity/ segmentation and cytoplasmic granularity, which are used to identify the cells. Cells with similar light scatter properties form a cluster in the scattergram and can be separated using advanced software algorithms. Some analyzers only use two angles of light, whereas others use multi-angle optical scatter analysis.
The more detectors (positioned at various angles) that are used to collect signals on each cell, the more information generated on the cellular characteristics, thereby increasing the accuracy of cell identification. The use of optical technology has led to the ability to provide five-part WBC differentiation and depending on the sophistication of the instrument, some current analyzers may report six-part WBC differentials, including immature granulocyte (IG) counts.10 Adding the capability of detecting a fluorescent signal further enhances the potential of optical technology. Fluorescent flow cytometry captures the light emitted from internal cellular components that are stained with a fluorescent dye as cells pass through the flow cell in front of the laser beam. Fluorescence is frequently integrated with multiple-angle optical light scatter methods to further improve the WBC differential subtype classification.
MAPSS technology
Multi-Angle Polarized Scatter Separation (MAPSS™) technology uses four light scatter detectors to determine various cellular features. The application of a depolarized light detector is a unique characteristic of this method and allows for specific identification of eosinophil granulocytes. The four detectors generate the following signals:- 0° or Axial Light Loss (ALL): related to size
- 0° to 10° Intermediate Angle Scatter (IAS): related to cellular complexity
- 90° Polarized Side Scatter (PSS): related to nuclear lobularity/segmentation
- 90° Depolarized Side Scatter (DSS): related to eosinophil granules.
These signals correlate with morphological characteristics that can be determined visually under the microscope from a stained slide. Various combinations of these four measurements are used to classify the WBC subpopulations and provide morphological flagging. Fluorescent flow cytometry is used to detect NRBCs based on DNA staining when additional reagents and a detector for fluorescence signal is added.
Hematology in the future
There have been numerous promising directions that could positively impact the future of the automated WBC differential. The International Council for Standardization in Hematology (ICSH) has proposed a new reference method for the leukocyte differential based on the use of monoclonal antibodies (mAbs) and multicolor flow cytometry.11 This would provide manufacturers with accurate and reproducible WBC classification for the development and improvement of automated technologies.Another opportunity for improvement is the incorporation of additional light scatter measurements in the characterization of blood cells, with the aim of more accurate classification of WBC subpopulations and potential identification of immature or pathological cell types. For example, advanced MAPSS technology can enable the differentiation of seven subpopulations of nucleated cells including neutrophils, lymphocytes, monocytes, eosinophils, basophils, IGs (including metamyelocytes, myelomyelocytes and promyelocytes), and NRBCs (if present), by utilizing seven light scatter detectors and fluorescent flow cytometry.
References:
- Dutcher, TF. Automated Leukocyte Differentials: A Review and Prospectus. Laboratory Medicine. 1983:14,483-487.
- Pierre, RV. Peripheral Blood Film Review. Clinics in Laboratory Medicine. 2002:22,279-297.
- Kickler, TS, Rothe, M, et al. Improving platelet transfusion therapy using the ImmunoPLT method on the CELL-DYN 4000. Laboratory Hematology. 1998:4,80-87.
- Terstappen LWMM, Mickaels R, et al. Increased Light Scattering Resolution Facilitates Multidimensional Flow Cytometric Analysis. Cytometry, 1990:11,510-512.
- Barnes PW, McFadden SL, et al. The International Consensus Group for Hematology Review: Suggested Criteria for Action Following Automated CBC and WBC Differential Analysis. Laboratory Hematology. 2005:11,83-90.
- Rumke CL. The statistically expected variability in differential leukocyte counting. Differential leukocyte counting: CAP conference/Aspen 1977. 1978; 39-46.
- Clinical and Laboratory Standards Institute (CLSI). Validation, Verification, and Quality Assurance of Automated Hematology Analyzers; Approved Standard. Second Edition. H26-A2. Wayne, PA: CLSI; 2010
- Robinson, P. Wallace H. Coulter: Decades of Invention and Discovery. Cytometry. 2013: 832,424-438.
- Harris N, Kunicka J, Kratz A. The ADVIA 2120 hematology system: flow cytometry-based analysis of blood and body fluids in the routine hematology laboratory. Lab Hematol. 2005;11(1): 47-61.
- Ali Ansari-Lari M, Kickler T, Borowitz M, Immature Granulocyte Measurement Using the Sysmex XE-2100: Relationship to Infection and Sepsis. Am J Clin Pathol. 2003; 120:795-799.
- Roussel M, Davis BH, Tierry Fest and Brent L. Wood (2012) Toward a reference method for leukocyte differential counts in blood: Comparison of three flow cytometric candidate methods. Cytometry Part A: Vol. 81A, No. 11, pp. 973-982.

