Developing Stem Cell Therapy Solutions for Liver Disease

Complete the form below to unlock access to ALL audio articles.
Regenerative medicine is a promising area of research which develops methods to regrow, repair or replace damaged cells, organs or tissues.
HepaTx is a small California-based company, which is developing regenerative medicine solutions for life-threatening liver disease.
Heptatx grew from Stanford University and is powered by Eric Schuur, PhD (President and CEO), Mark Chao, PhD (Vice President of Clinical Development) and Harini Chakravarthy, PhD (Stem Cell Scientist).
Backed by a strong advisory board (including Gary Peltz MD, PhD, Stanford Anesthesiology, consultant Marie Csete, MD, PhD, and Shannon Dahl, PhD, Chief Scientist at Cell Care Therapeutics), the team is looking forward to seeing their research transition to the clinic.
To learn more, we spoke with Eric Schuur, President and CEO of HepaTx.
How did HepaTx come about?
The HepaTx co-founders met through a translational research training program offered by Stanford University’s Office of Technology Licensing. This program, the Innovation Farm Teams (or iFarm) program teamed up Stanford-affiliated individuals to develop a commercialization plan for one of Stanford’s unlicensed technologies. We reviewed 1800 technologies that Stanford had available and chose the one that eventually became the foundation for HepaTx to work on.As we worked through developing a commercialization plan for the technology, which is a way to use stem cells to treat liver failure, it became apparent to us that this could be a game-changing treatment for patients with liver failure. Dr. Peltz and his colleagues had done a great job of generating proof-of-concept data. It was apparent that the next logical step was to take the technology into commercialization and the best way to accomplish that was to found a company to do that development. Hence, HepaTx was created.
How do you hope your work will benefit people with liver disease?
The liver is an irreplaceable organ, so people with inadequate liver function suffer a variety of debilitating conditions and consume large amounts of medical care. Right now, liver transplant is the only treatment that changes the course of the disease for these patients and liver transplants are severely limited in number. The number of people who would benefit from a liver transplant exceeds the number of transplants available by 10-fold to 20-fold.Patients arrive at this medical state from many different causes, including hepatitis, alcohol ingestion, metabolic disease, autoimmune disease, and other etiologies. Our strategy for treating these patients with late-stage disease will apply to all of these patients.
Our goal is to provide an alternative treatment to transplant that can help these patients live normal lives. By developing a cell therapy that can be infused, we will be able to offer a therapeutic option to many of these patients who are not able to or are not eligible for a liver transplant.
Is there a specific type of liver disease that you are working on?
Ultimately, we believe that this therapy will help patients with many different types of liver disease. However, the first group of patients that we hope to help are those with acute liver failure because we think we can help these patients soonest. These patients have a high unmet medical need: nearly 30% will die within 1-2 months. We believe the path to a treatment that will alter the clinical outcome of these patients is shortest. The technologies we develop and knowledge we acquire on this path will accelerate our progress toward therapies for patients with more chronic forms of liver failure and other forms of liver disease.Can you give us a brief overview of the steps involved in hepatocyte production in your lab?
Our raw material is stem cells that are obtained from fat tissue. We obtain this tissue from normal donors at plastic surgery practices. We transport the material to our facility where we isolate the stem cells. We isolate the stem cells from the fat tissue by incubating the tissue with collagenase to release the cells, then differential centrifugation to separate the stem cells from the adipocytes. After isolation, these stem cells are used for manufacturing hepatocytes.To manufacture the liver cells from the stem cells we use technology that was invented in the lab of Dr. Gary Peltz at Stanford University. Using this technology, we expose the cells to culture conditions and growth factors that cause the stem cells to differentiate into liver cells. Over the course of about 2 weeks we place the stem cells in suspension culture, then expose them sequentially to defined media that induce hepatocyte differentiation through two stages. These cells can then be used to transplant into recipients with damaged livers, where they home to the liver and replace lost liver cells.
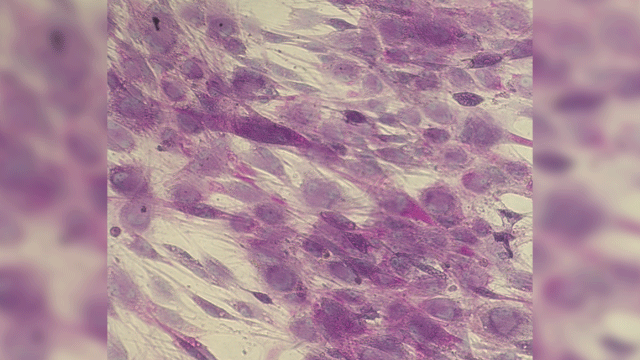
Manufactured hepatocytes. Credit: HepaTx
How do you assess the performance of manufactured liver cells?
We use a variety of phenotype measurements and functional assays in culture before infusing the cells into animals. These include measuring expression of hepatocyte-specific genes, such as albumin and cytokeratin 8/18, and measuring hepatocyte-specific functions, such as glycogen synthesis and metabolism of drug molecules by cytochrome enzymes.In animals we can measure the function of the cells directly by looking for them in liver tissue with antibody assays and also indirectly by measuring typical clinical assays for liver function, such as ALT (alanine aminotransferase) and ammonia levels.
What items need to be ticked off your to-do list, before this technique can be made available to patients?
We will need to demonstrate for regulatory authorities the efficacy of the cells in an animal model of liver failure. In addition to this critical test in our liver failure model, we will need to demonstrate biodistribution of the cells following infusion and test for tumorigenicity in animals.The other area that we will need to complete work in is manufacturing. We will demonstrate the ability to manufacture the cells with quality parameters acceptable to regulatory authorities. That will require validation of specific assessments that we use to measure the production process. This forms the critical framework for production of clinical-grade cells for administration into humans.
Once we have done these things, we will apply for permission to test the cells in patients with acute liver failure.
Eric Schuur was speaking with Michele Wilson, PhD, Science Writer for Technology Networks.


