Injectable Discogenic Cell Therapy Developed to Alleviate Back Pain
Complete the form below to unlock access to ALL audio articles.
Lower back pain affects approximately 25 million adults in the U.S. alone and can be incredibly debilitating.
Degenerative disc diseases are a major contributor to low back pain, and current treatment options are lacking. We spoke with leaders of DiscGenics, CCRM, and GE Healthcare Life Sciences, three companies which have joined forces to tackle this global health concern.
Flag Flannagan, Michael May and Phil Vanek give us an overview of current challenges related to degenerative diseases of the spine, and tell us how they hope to alleviate pain and restore function for millions.
Image adapted from: Discgenics, Inc.
What are some limitations of current treatment approaches for degenerative disc disease?
Flagg Flanagan, Chairman and CEO, DiscGenics:
Degenerative disc disease (DDD) is a painful, chronic and progressive disease that is characterized by inflammation and breakdown of tissue within the intervertebral disc.
In the earlier stages, current treatment options are limited to physical rehabilitation programs and pain management approaches that are typically not intended for long-term use. Opioids, epidural steroid injections and radiofrequency ablation are a few examples of these.
In the more advanced stages, oftentimes a patient’s only option is costly surgical intervention to remove the painful disc(s), fuse two or more vertebral bones together, and/or replace bone or tissue altogether. However, back surgeries often have limited success and may result in subsequent adjacent level degeneration.
Ultimately, the challenge is that while chronic low back pain is the leading cause of disability worldwide, these options offer only temporary pain relief without actually healing the root cause of the pain: the degenerated disc.
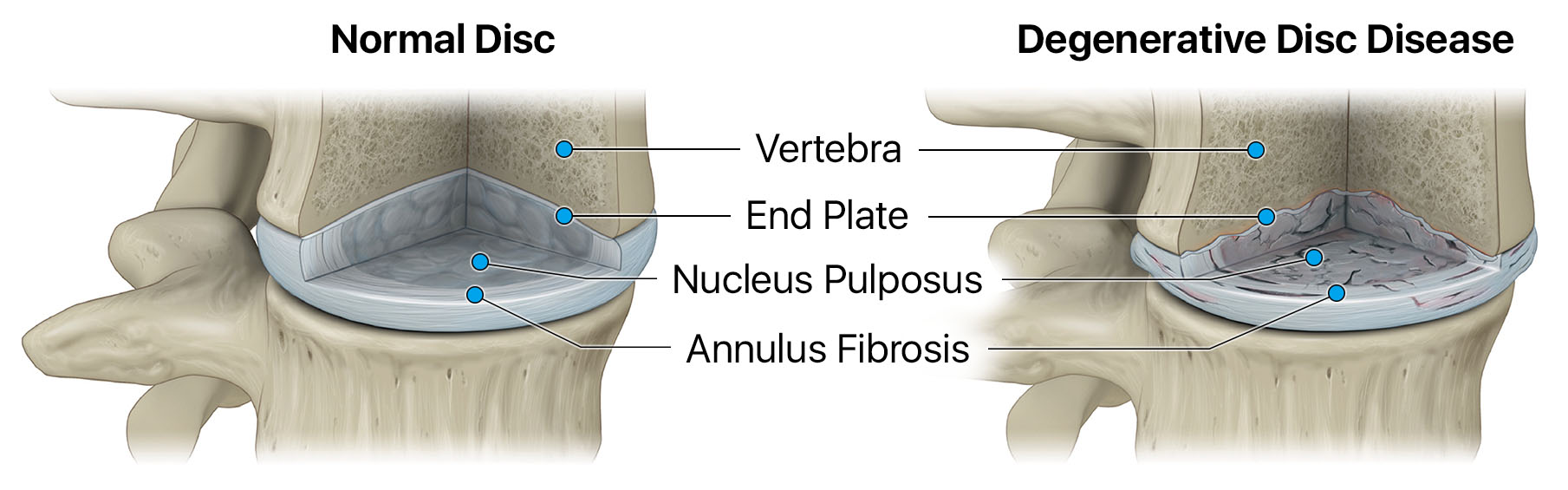 Degenerative disc disease is characterized by inflammation and the breakdown of tissue within intervertebral discs of the spine. Credit: DiscGenics, Inc.
Degenerative disc disease is characterized by inflammation and the breakdown of tissue within intervertebral discs of the spine. Credit: DiscGenics, Inc.
Can you tell us about IDCT and the advantages it could offer patients?
Flagg Flanagan: IDCT is an off-the-shelf, non-surgical approach to healing patients with DDD.
DiscGenics has a proprietary manufacturing process by which we engineer donated intervertebral disc tissue into highly specialized therapeutic progenitor cells for injection into the degenerated disc.
Preclinical studies have shown that these cells, known as Discogenic Cells, possess the key regenerative and anti-inflammatory properties of mesenchymal stem cells (MSCs) as well as the ability to normalize disc architecture, regenerate new disc tissue and restore disc height when injected into a degenerated disc.
Earlier this year, DiscGenics initiated a first-in-human, 60-subject Phase I/II clinical trial of IDCT in patients with mild to moderate DDD.
If IDCT demonstrates similar results in human subjects, the outcome could be reduced pain and disability, potentially revolutionizing the way we treat patients with DDD by bypassing many mid-stage treatments and potentially eliminating non-restorative, late-stage surgeries.
It’s important to note that DiscGenics is the only company to utilize an allogeneic cell therapy derived from disc cells to treat the disc.
Because Discogenic Cells begin as disc cells, they already know how to behave in a complex disc environment and are believed to have many advantages over other investigational approaches that use unspecialized cells from sources like bone marrow or adipose fat tissue that must first adapt to the disc environment before even attempting to introduce a therapeutic effect.
IDCT is an investigational allogeneic (donor-derived), injectable (non-surgical) cell therapy that utilizes proprietary Discogenic Cells to treat degenerative disc disease.
How did the partnership between DiscGenics, CCRM and GE Healthcare come about, and what roles are each group playing?
Michael May, President and CEO, CCRM: CCRM and GE Healthcare are collaborators in the Centre for Advanced Therapeutic Cell Technologies (CATCT), an initiative launched in January 2016 with CA$40 million (matched funding) from GE Healthcare and the Government of Canada, via the Federal Economic Development Agency for Southern Ontario.
CCRM and GE are developing advanced manufacturing solutions in cell and gene therapy around process development and production. In a short period of time, CATCT has made progress in addressing specific bottlenecks limiting commercial-scale manufacturing of cell and gene therapies, all with the aim of identifying, developing and adopting advanced manufacturing solutions to help customers produce better treatments for patients.
CATCT offers tailored contract services to cell and gene therapy developers.
For DiscGenics, CCRM and GE Healthcare are scaling and optimizing the manufacturing of its allogeneic injectable cell therapy: IDCT.
CATCT is conducting process, assay and media development. The work begins with isolating cells from donated intervertebral disc tissue and results in highly-specialized “Discogenic Cells” to address the complex environment of the degenerated disc.
This approach enables DiscGenics to introduce restorative progenitor cells to the damaged disc and offers a therapeutic option for millions suffering from the debilitating effects of back pain.
What hurdles do regenerative medicines face on the path to clinical translation, and how are partnerships such as this helping to overcome them?
Phil Vanek, GM of Cell and Gene Therapy Strategy at GE Healthcare Life Sciences: Regenerative medicine therapies face multiple hurdles throughout clinical translation and beyond to commercialization. To move the needle on a global scale with a significant number of patients, the industry has to overcome significant distribution and access challenges. The manufacturing process also is very complex and challenging, and that is where CCRM and GE Healthcare are having an immediate and meaningful impact through our work in providing process development services, training and modular manufacturing technologies.
CATCT is providing cell therapy companies with facilities, technologies and expertise to help establish manufacturing processes that can produce the large cell numbers required for clinical and commercial use.
Addressing these particular challenges, while necessary for therapy developers, it is a priority for the highly-skilled engineers and scientists in CATCT.
DiscGenics was an early adopter of the process development capabilities GE Healthcare and CCRM have built together and this will enable the company to move ahead as it progresses through clinical trials.
The therapy is the first case study to come out of the Centre for Advanced Therapeutic Cell Technologies (CATCT), a new fully active cell therapy research and process development hub in Toronto, which is a collaboration among Canada’s Centre for Commercialization of Regenerative Medicine (CCRM), GE Healthcare, and The Federal Economic Development Agency for Southern Ontario (FedDev Ontario).
Flagg Flanagan, Michael May, and Phil Vanek were speaking with Anna MacDonald and Michele Wilson, Science Writers for Technology Networks.



