Manipulating Cell Networks With Light – New Frontiers in Optical Microscopy

Complete the form below to unlock access to ALL audio articles.
A new optical microscope system called SIFOM (Stimulation and Imaging-based Functional Optical Microscopy) can stimulate multiple cells simultaneously by a holographic method and monitor cell activity after the stimulation using 3D measurements based on fluorescence holography.
This system has potential applications as a tool for the reconstruction of lost nerve pathways, constructing artificial neural networks, and food resources development. The concept of SIFOM and the feasibility check were published on November 1 in Optics Letter, one of the top journals of the optics and photonics research field.
So far, many optical microscopies such as phase contrast microscopy, fluorescence microscopy, multi-photon microscopy, and super-resolved fluorescence microscopy have been developed. Recent breakthroughs in optics technology have enabled scientists to visualize the ultrafine structure of cells and their functions even in vitro and in vivo.
We can now use the light to manipulate cell activity as in optogenetics by using channelrhodopsin or other related proteins (see figure 1). However, the present optogenetics-based light stimulation used to manipulate cell activity is too simple, using uniform exposure by LED or through optic fibers, so only a low level of cell manipulation is possible.
This study proposes a new optical microscope system called SIFOM (figure 2). The SIFOM consists of two sub-functions: 3D observation of cells and 3D stimulation of cells based on digital holography. This is the first microscope to be equipped with technology that can simultaneously carry out 3D observation and stimulation, and it has potential applications as a groundbreaking tool in the life sciences. Using high-speed scanless photography, this technology makes it possible for us to gain information about multiple events occurring in 3D space within a very short time frame.
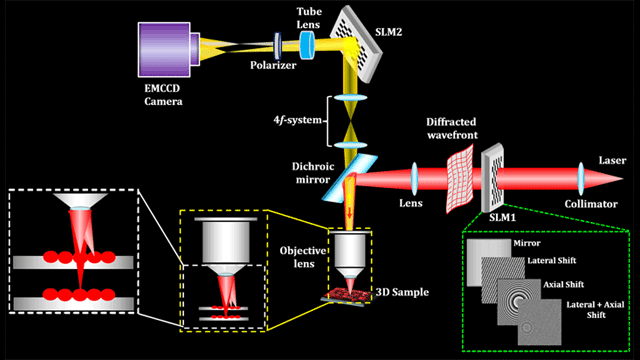
Figure 2: Concept of the SIFOM for making cell manipulation technology combining 3D fluorescence observation and 3D stimulation. Image credit: Kobe University
As a verification experiment, the team used lung cancer cells and fluorescent beads about 10 micrometers in size. They recorded a fluorescent hologram in a defocused state from the focal position in the direction of depth and achieved reconstruction of both the cells and the fluorescent beads (figure 3).

Figure 3: 3D recording of lung cancer cells; (a) 2D fluorescent observation, (b) a fluorescent hologram when one cell is selectively extracted, (c) a hologram when moved to a depth of 80 µm, (d) a reconstruction of (c), (e) after extracting two cells and exposing to light stimulation, (f) a hologram when moved to a depth of 80 um, (g) reconstruction of (f). Image credit: Kobe University
The study was carried out by a multi-institution interdisciplinary collaborative research team led by Professor Hiroaki Wake (Kobe University, Graduate School of Medicine) and Professor Osamu Matoba (Kobe University, Graduate School of System Informatics) in collaboration with Professor Yasuhiro Awatsuji (Kyoto Institute of Technology, Faculty of Electrical Engineering and Electronics) and Professor Yoshio Hayasaki (Utsunomiya University, Center for Optical Research and Education).
During the verification experiment, they were able to observe light stimulation for a maximum of five cells at one time. The maximum number of stimulated cells is determined mainly because there is insufficient light power for stimulation. In 2D (two-dimensional) space, it is expected that simultaneous light stimulation is possible for over 100 cells, and in the future, the team aims to expand the stimulation depth to a few hundred micrometers using two-photon stimulation.
In order to observe living cells, there is a limit to the power of the fluorescence to avoid damaging cells, so high-sensitivity measurements are required. The team aims to overcome these issues and prepare the new optical microscopy system for practical use. Professor Matoba comments, “We have a research grant from JST CREST Grant Number JPMJCR1755, Japan to fabricate a SIFOM and then apply it to further development of neuroscience. We will collaborate with companies to introduce the new optical microscope into the commercial market”.
This article has been republished from materials provided by Kobe University. Note: material may have been edited for length and content. For further information, please contact the cited source.
Reference:
Quan, X., Kumar, M., Matoba, O., Awatsuji, Y., Hayasaki, Y., Hasegawa, S., & Wake, H. (2018). Three-dimensional stimulation and imaging-based functional optical microscopy of biological cells. Optics Letters, 43(21), 5447. doi:10.1364/ol.43.005447

