Regulation of Cell Orientation and Shape for Tissue Morphogenesis

Complete the form below to unlock access to ALL audio articles.
A collaborative research group led by Kumamoto University has identified a new control system which regulates the morphology and orientation of cells, as published in EMBO Reports.
Cells that make up animal tissues must have proper form and an ordered arrangement. This behavior is determined by cell polarity where the opposite ends of a cell, such as up/down or front/back, have different properties.
For example, the hair that grows on the surface of Drosophila (fruit fly) wings is aligned in a particular direction because it grows only from the outer part of each cell that forms the surface of the wing. This is called planar cell polarity.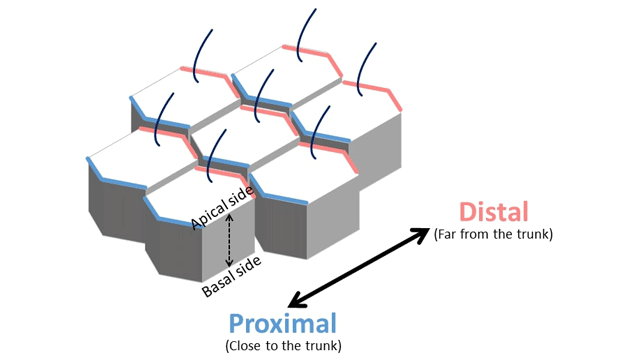 Epithelial cells constituting the epithelial tissue have two cell polarities. Credit: Dr. Koji Kikuchi
Epithelial cells constituting the epithelial tissue have two cell polarities. Credit: Dr. Koji Kikuchi
Tissues will not form correctly if cell polarity formation is impaired, and malformation can lead to the development of various diseases. Furthermore, maintaining cell polarity is necessary for preserving tissue homeostasis. Failure of this mechanism can lead to carcinogenesis and malignant cancer.
Since the formation of cell polarity involves a change in cell morphology, the cytoskeleton also changes – as the cytoskeleton determines the morphology of the cell.
Changes in cytoskeleton dynamics are induced by intracellular messengers (signaling molecules), and it has been suggested that the cytoskeleton not only receives a signal at the time of kinetic changes, but may also produce feedback through some form of signal transduction.
However, the mechanism linking cytoskeletal dynamics and the respective signaling mechanisms has not yet been clarified.
Researchers from Kumamoto University studied the Wnt5a signaling pathway, which controls the front-rear polarity (i.e., the cell shape during movement) of cells derived from human cervical cancer.
They found a microtubule-associated protein, Map7/7D1, which links the Wnt5a signaling pathway and microtubule dynamics by associating with Dishevelled (Dsh, or Dvl in mammals) protein, a transmitter in the Wnt5a signaling pathway.
The Wnt5a signaling pathway is already known to be essential for planar cell polarity formation in epithelial cells.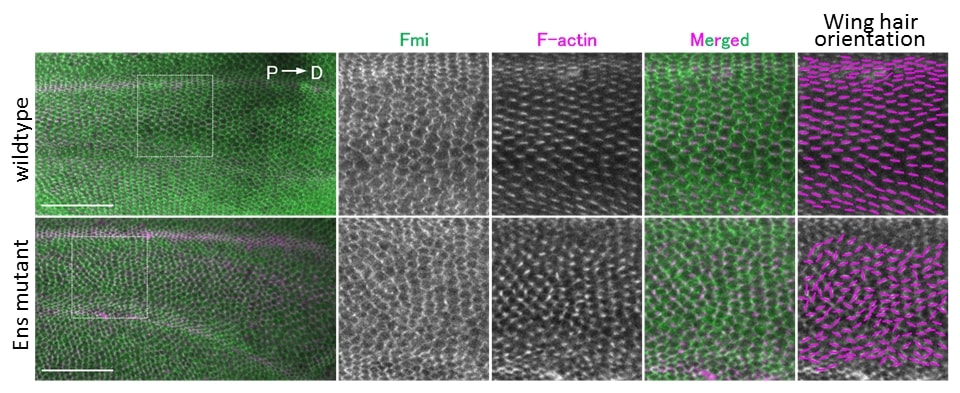
Planar cell polarity formation was evaluated by analyzing the direction of wing hair using wing epithelial tissue of fruit fly pupa. With mutants of MAP7/7D1 (Ens in Drosophila), the direction of winged hair was not aligned, and abnormality of PCP was observed (bottom right). Credit: Dr. Koji Kikuchi
In this study, researchers examined the function of Map7/7D1 during polarity formation using Drosophila wing and mouse oviduct epithelial tissues.
Results showed that the microtubule-associated proteins Map7/7D1 and Dvl in Drosophila, Ens and Dsh, bind together, and that Ens controls the localization of Dsh to the distal side of the cell.
Furthermore, Map7/7D1/Ens showed planar-polarized distribution at the ovary/proximal side in epithelial cells of mouse oviducts and Drosophila pupal wings, respectively.
Since the behavior and properties in cells (such as localization patterns and binding with Dvl/Dsh) are conserved, the researchers suggest that the functions of Map7/7D1/Ens are conserved and inherited similarly in many different species during planar cell polarity formation.
This article has been republished from materials provided by Kumamoto University. Note: material may have been edited for length and content. For further information, please contact the cited source.
Reference:
Kikuchi, K., Nakamura, A., Arata, M., Shi, D., Nakagawa, M., Tanaka, T., . . . Nakanishi, H. (2018). Map7/7D1 and Dvl form a feedback loop that facilitates microtubule remodeling and Wnt5a signaling. EMBO Reports, 19(7). doi:10.15252/embr.201745471

