Mitosis vs. Meiosis: Key Differences, Chart and Venn Diagram

Complete the form below to unlock access to ALL audio articles.
Introduction
In order for organisms to grow, cells have two options: they must either replicate themselves to create more cells, or the cells themselves must expand in volume. In humans, tissues such as the skin and blood contain cells that are actively dividing, whilst other tissues such as fat contain cells that expand (good if you need energy for winter, bad if you are trying to fit into some expensive jeans). Other cells, such as neurons, will never divide again once they are terminally differentiated; they are post-mitotic.
In the process of replicating themselves, cells have another choice: do they want to make an identical copy and be left with two cells? Or do they want to make four “half-copies”, in preparation for sexual reproduction, where their genetic content will be made whole again by the process of fertilization? This choice is the choice between mitosis and meiosis.
Mitosis and meiosis
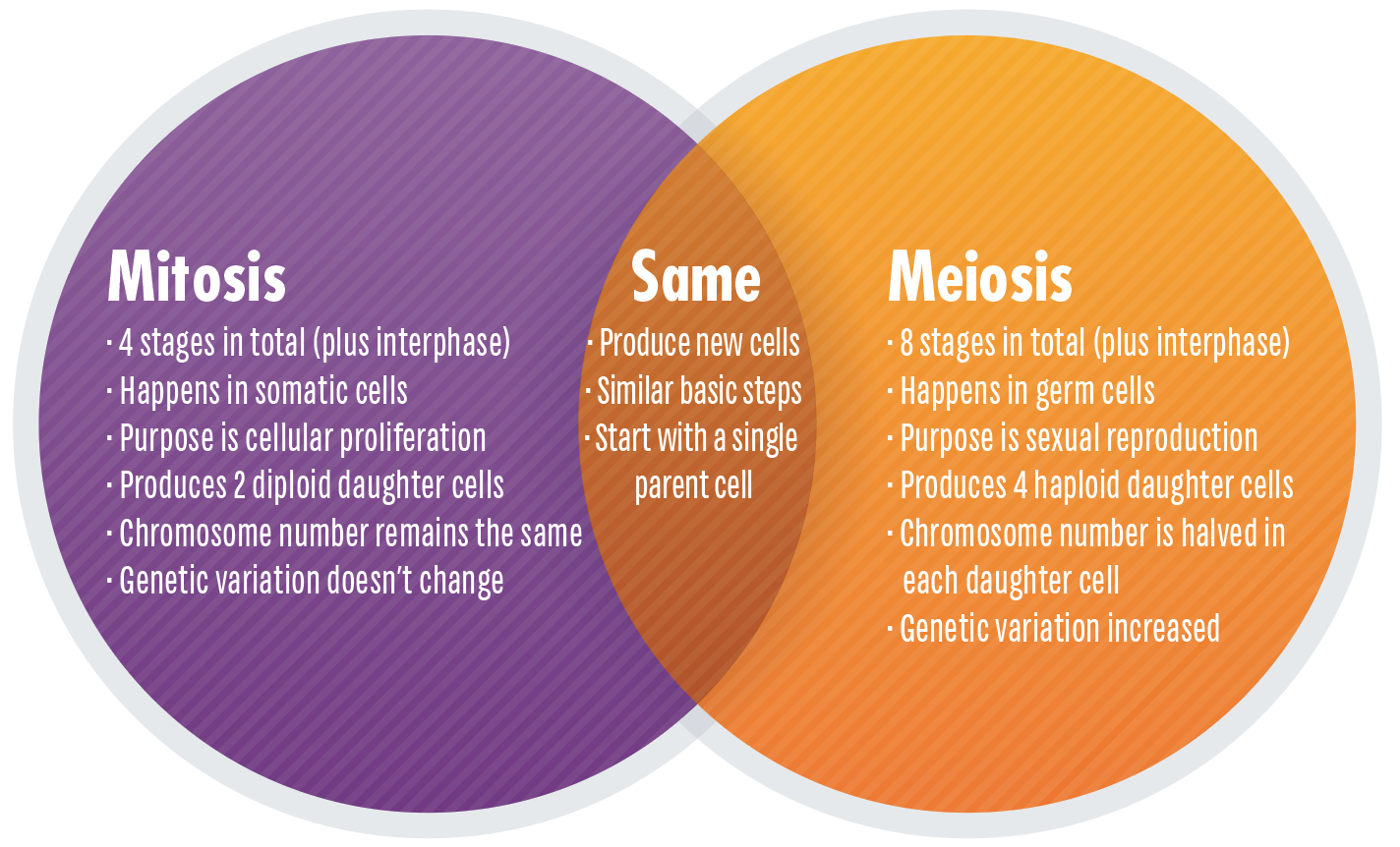
There are two types of cell division: mitosis and meiosis. Mitosis is the process by which body cells divide and create copies of themselves for growth and repair. In meiosis, the new cells have half the genetic material of the parent cell and is the process by which egg and sperm cells are formed.
Difference Between Mitosis and Meiosis
This article will explore the characteristics of both kinds of cell division, shining a light on how they are similar and in which aspects they are crucially distinct. We will also explore the research into these processes and how cell division might go awry to cause disease states such as cancer and Down’s Syndrome.
Mitosis vs. Meiosis: Overview and commonly asked questions
Mitosis | Meiosis |
| What is the purpose of this process? | |
| In a unicellular organism, the purpose of mitosis is to proliferate as a species. In a multicellular organism, the purpose can be to grow during development, or to repair or regenerate a damaged tissue, for example. | To create gametes with only one copy of the organism’s genetic information, in preparation for sexual reproduction. Various steps in meiosis create opportunity for genetic diversity in the daughter cells. This is the raw substrate for evolution. |
| What is the outcome of this process? | |
| Two diploid cells with identical genetic information. | Four haploid cells with different genetic information. |
| Which organisms perform this process? | |
| Mitosis is performed by unicellular and multicellular eukaryotes. Bacteria have their own version of mitosis called “binary fission”. This is distinct from meiosis as bacteria typically have one circular chromosome, which is not contained within a nucleus, like eukaryotic chromosomes. | Only organisms which perform sexual reproduction. Archaea and bacteria do not do this, so it might be tempting to think that unicellular organisms do not sexually reproduce. However, there are exceptions; budding yeast will form haploid spores under nutritional deprivation. |
| How long does this process take? | |
| Mitosis is usually shorter than meiosis. The process can take over 10 hours for mammalian cells in culture [2], budding yeast can take ~80 minutes to complete a cell cycle [3], whilst bacteria can divide every 20 minutes. | Meiosis has various timescales in different organisms, which can be affected by several factors including temperature and environment of the organism, and the amount of nuclear DNA. The process lasts 6 hours in yeast but can last more than 40 years in human females, due to a developmental hold at prophase I, until ovulation. Other examples are 1-2 days in male fruit flies and ~ 24 days in human males. [1] |
| What is an example of a disease caused by an error in this process? | |
| Uncontrolled mitosis occurs in cancer, where either genes that stop cell division (tumor suppressors) are switched off, or genes that encourage cell division (oncogenes) are overactive. | Errors in meiosis can lead to the wrong number of chromosomes ending up in germ cells, this is called aneuploidy. This can trigger miscarriage, but is occasionally tolerated. One example is Down’s syndrome, caused by trisomy 21. Another example is Klinefelter syndrome, where XY males have an additional X chromosome. |
| Etymology? | |
| Mitosis is the Greek word for thread, after the thread-like chromosomes that can be seen under the microscope in dye-stained cells during cell division. | Meiosis means a “lessening” in Greek. This refers to the outcome of meiosis, where the genetic information in each new cell is halved. |
| First described by? | |
| Walther Flemming in his 1882 work “Cell substance, nucleus and cell division.” [5] | Oskar Hertwig described the fusion of egg and sperm in the transparent sea urchin egg in 1876. [4] |
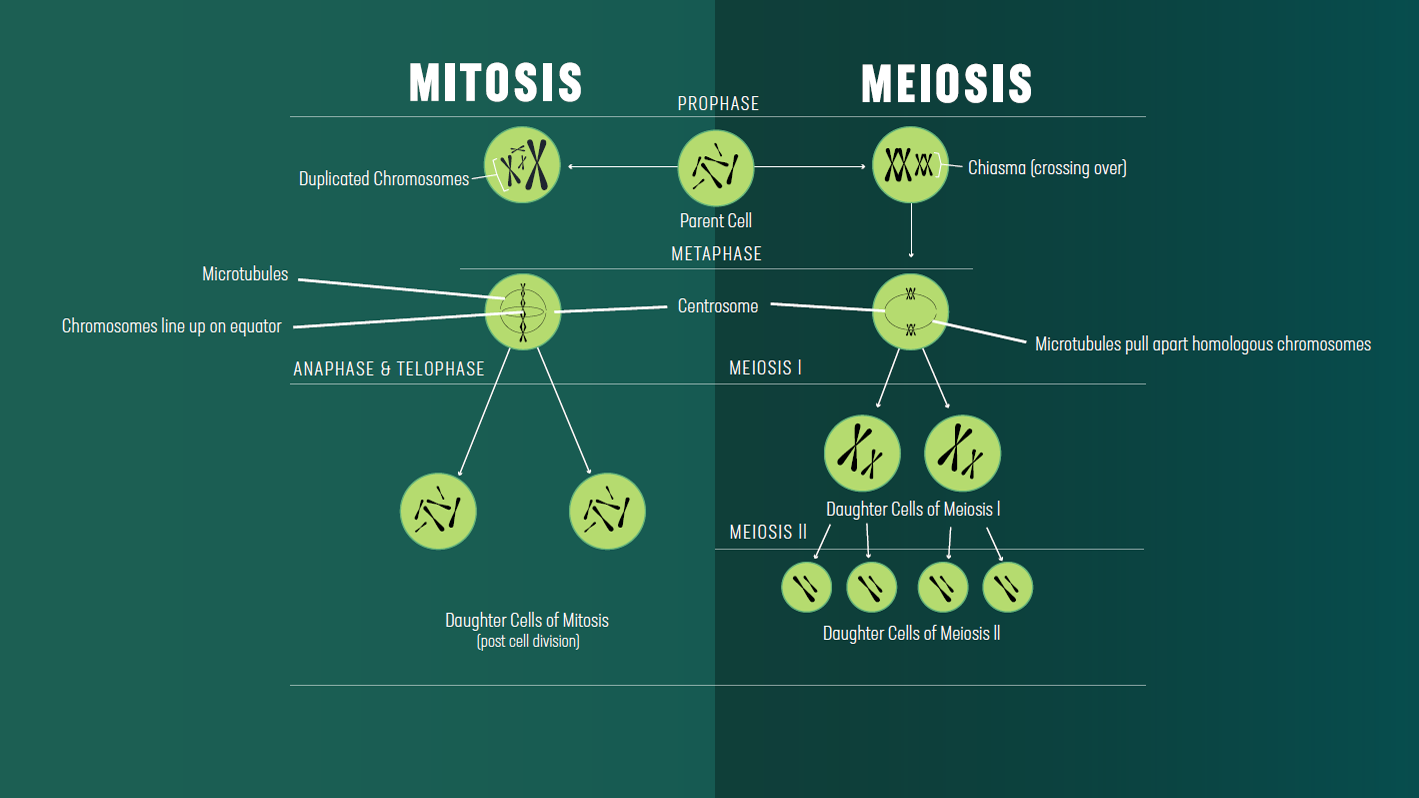
The stages of mitosis vs. meiosis
| Summary | |
| Meiosis and mitosis both have a prophase, metaphase, anaphase, telophase and cytokinesis. | |
| In meiosis, prophase, metaphase, anaphase and telophase occur twice. The first round of division is special, but the second round is more like mitosis. | In mitosis, prophase, metaphase, anaphase and telophase occur once. |
| Prophase | |
| Chromosomes condense and the centrosomes begin to form an early spindle. | |
|
|
| Metaphase | |
| In metaphase II of meiosis, and metaphase of mitosis, chromosomes line up along the metaphase plate due to the action of microtubule spindle fibers emanating from the centrosomes located at opposite cell poles. These fibers are attached to the chromosomes by kinetochores at the centromeres of the chromosomes. | |
|
|
| Anaphase | |
| In anaphase, chromosomes are split to opposite poles of the cell. | |
|
|
| Telophase | |
| A nuclear membrane reforms around the newly separated chromosomes, which begin to uncoil, becoming less condense. The spindle microtubules disassociate. Each daughter cell will inherit one centrosome. | |
| Cytokinesis | |
| The cell plasma membrane pinches, to leave two daughter cells with separate plasma membranes. | |
|
|
Memory Tricks
Mnemonics are also helpful, for example a useful mnemonic to remember the order of the steps in mitosis is “I Prefer Mating At Teatime” – Chamillionaire.

| Mitosis Stage | Chromosomes |
| Interphase | Are uncondensed but are still organized. The entire genome is replicated to create two identical semi-conserved copies of each chromosome. |
| Prophase | Condense. Duplicated chromosomes are called sister chromatids. |
| Metaphase | Align along the metaphase plate, the midpoint between the two centrosomes. Sister chromatids are joined at the centromere by proteins that form a structure called a kinetochore. |
| Anaphase | Cohesin is cleaved at the centromere of chromosomes, resulting in sister chromatids being pulled to opposite poles of the cell. |
| Telophase | Chromosomes begin to uncoil, becoming less condensed. |
| Cytokinesis | Chromosomes have returned to their interphase structure. This is a topic of much research, but it seems as though each chromosome occupies its own territory within the nucleus. |
| Mitosis Stage | Centrosomes |
| Interphase | The centrosome is duplicated. |
| Prophase | Microtubules begin to form an early mitotic spindle between the duplicated centrosomes. |
| Metaphase | The two centrosomes are now located at opposite poles of the cell. |
| Anaphase | Microtubules emanating from the centrosomes shrink as the tension holding the chromosomes at the metaphase plate is broken by cohesin cleavage. |
| Telophase | The centrosomes remain segregated to opposite sides of the cell. Each daughter cell will receive one centrosome comprised of two centrioles. |
| Cytokinesis | Centrosomes signal to the cell that it is okay to proceed with cytokinesis. Research shows that cells where centrosomes are destroyed with a laser beam cannot undergo cytokinesis. |
| Mitosis Stage | Nuclear Membrane |
| Interphase | Intact. |
| Prophase | Intact. |
| Metaphase | In higher eukaryotes like vertebrates, by the time metaphase occurs the nuclear envelope has broken down. This is caused by phosphorylation of nuclear lamin proteins. |
| Anaphase | Broken down. |
| Telophase | A nuclear envelope reforms around the chromosomes in each daughter cell. |
| Cytokinesis | Intact. |
| Mitosis Stage | Plasma Membrane |
| Interphase | Intact. |
| Prophase | Intact. |
| Metaphase | Intact. |
| Anaphase | Intact. |
| Telophase | Intact. |
| Cytokinesis | Pinches to form two separate membranes around the two daughter cells. |