Measuring the T Cell Immune Response to COVID-19

Complete the form below to unlock access to ALL audio articles.
Learning more about the strength of a person’s immune response to SARS-CoV-2 could help scientists to better understand immunity to the virus and the reasons why responses vary between individuals.
Oxford Immunotec has recently released a new tool that can measure the T cell immune response to SARS-CoV-2, the T-SPOT Discovery SARS-CoV-2 kit. To learn more about the T-SPOT technology, discover how it can help to assess the strength of a person’s immune response to SARS-CoV-2, and why this is important, Technology Networks spoke with Andrew Makin, VP Medical Affairs, Oxford Immunotec.
Anna MacDonald (AMD): What do we know so far about immunity to SARS-CoV-2?
Andrew Makin (AM): Currently, not much is known about immunity to SARS-CoV-2. It is encouraging that it looks like most people do develop an innate and adaptive immune response to the virus, which indicates that previous infection could potentially offer some level of protection against re-infection, but as SARS-CoV-2 is so new this remains to be proven.
It can be anticipated that an initial infection will lend people some level of immunity for some amount of time. But there are still many unknowns around robustness of response, duration of immunity and building blocks of that immunity – antibodies, cells, and other markers in a person’s blood. Antibody responses and T cell mediated responses have been detected in convalescent populations, however we still do not know for certain that this will confer any protective immunity1.
AMD: What can measuring the T cell immune response to SARS-CoV-2 tell us?
AM: Measuring the T cell response to SARS-CoV-2 infection can tell us whether there are any T cells in a patient sample that recognize the SARS-CoV-2 specific peptides used in the assay. If an individual has SARS-CoV-2 reactive T cells, this may be indicative of prior exposure to the virus. This is a fast-moving field. Researchers know now that T cells play a role in eliminating SARS-CoV-2. This could potentially have important implications in diagnosis, prognosis and long-term immunity to SARS-CoV-2.
AMD: Can you give us an overview of the T-SPOT technology?
AM: The T-SPOT technology is a proven technology. It is approved for clinical use to detect TB infection in over 60 countries. With over 20 million clinical tests manufactured since release, the T-SPOT technology:
- Uses a standardized sample prepared from peripheral blood which:
- Reduces the influence of factors that might affect results, such as other treatments
- Standardizes cell numbers in the test to normalize for cell number variations between samples
- Allows the number of responding T cells to be enumerated for a precise assessment of the T cell response
- Is able to maintain performance, even in samples from immunosuppressed individuals
- Can be run in high-volume labs, enabling large testing programs to be rolled out
This pioneering technology has been described in over 700 peer-reviewed publications showing the test’s excellent performance in a wide variety of clinical and epidemiologicalsettings including HIV positive, Anti-TNFα treated patients, healthcare worker screening and new entrant screening to mention a few.
Peripheral Blood Mononuclear Cells (PBMCs) are separated from a blood sample, washed and counted. A specific number of PBMCs and the antigens specific to the disease or condition of interest are then added to the wells of a microtiter plate to which antibodies to interferon-gamma (IFN-gamma) are bound.
The T cells which have previously encountered the specific antigen in vivo (due to infection) will respond to the antigens in vitro by secreting IFN-gamma. The IFN-gamma secreted by the T cells of the subject is captured by the anti-IFN-gamma antibodies coated to the base of each well. The numbers of individual reacting T cells are enumerated through visualizing the footprint of each T cell by this secretion of IFN-gamma.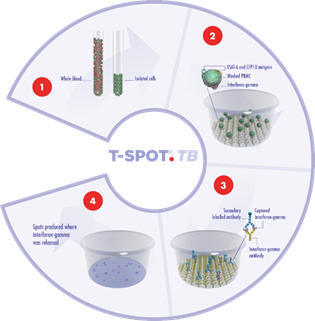
1. A blood sample is collected using routine phlebotomy and a standard blood collection tube from which a subset of white blood cells, known as peripheral blood mononuclear cells, are isolated. The cells are washed, counted and normalized to create a standard cell suspension.
2. A standard number of cells are added into specially designed plates and stimulated with antigens specific to the disease under study. Cells responding to these antigens release a chemical messenger known as a cytokine.
3. Cytokine antibodies are used to directly capture the cytokine as it is released by the cells. A secondary labeled antibody is added and binds to the captured cytokine.
4. A detection reagent is added and reacts with the secondary labeled antibody. This reaction produces spots, which are a footprint of where the cytokine was released. Spots are then enumerated.
AMD: How soon can T cells be detected after infection?
AM: How soon T cells can be detected after infection remains to be seen. However, it has been reported that T cells have been detected in individuals infected with SARS-CoV-2 approximately 1 week after symptom onset2,3. This early T cell detection could be important. We know that most people infected with SARS-CoV-2 can display an antibody response between day 10 and day 21 after infection4-6.
AMD: How does the test compare to other methods of serology testing, such as detecting IgG or IgM antibodies? What advantages does it offer?
AM: The T-SPOT Discovery SARS-CoV-2 kit is intended to be used to assess the cell-mediated immunity (CMI) to SARS-CoV-2 using the T-SPOT technology platform (ELISPOT) with viral peptide pools derived from SARS-CoV-2.
Several limitations have been reported with the measurement of antibody mediated immunity. Importantly, antibodies may not be detected after infection if the antibody response is delayed, or there is no significant antibody response7,8. As such, understanding CMI may help to better understand the immune response to SARS-CoV-2 infection, especially in individuals who do not produce a measurable antibody response.
The T-SPOT technology also potentially allows for the enumeration of the SARS-CoV-2 reactive T cells, it may provide additional information on immunity to SARS-CoV-2 and possible protection against re-infection. This, however, is yet to be proven and T-SPOT Discovery SARS-CoV-2 remains a “research use only” test.
References
1 Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020: 181; 1-13.
2 Thevarajan I, Nguyen TH, Koutsakos M, et al. Breadth of concomitant immune responses underpinning viral clearance and patient recovery in a non-severe case of COVID-19. medRxiv 2020: 2020.0220.20025841.
3 Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype of SARS-CoV-2-specific T-cells in COVID-19 patients with acute respiratory distress syndrome. medRxiv 2020: 2020.04.11.20062349.
4 Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. medRxiv. 2020:2020.03.02.20030189.
5 OKBA NMA, Muller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, et al. SARS-CoV-2 specific antibody responses in COVID-19 patients. medRxiv. 2020:2020.03.18.20038059.
6 Liu W, Liu L, Kou G, Zheng Y, Ding Y, Ni W, et al. Evaluation of Nucleocapsid and Spike Protein based ELISAs for detecting antibodies against SARS-CoV-2. medRxiv. 2020:2020.03.16.20035014.
7 Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 2020: 2020.03.30.20047365.
8 World Health Organization, Scientific Briefs. "Immunity passports" in the context of COVID-19.
T-SPOT® Discovery™ SARS-CoV-2 is for research use only: Not for use in diagnostic procedures
Andrew Makin was speaking to Anna MacDonald, Science Writer, Technology Networks.


