Uncovering the Food Chain’s Hidden Dangers: Low Level Dioxin Quantification by GC-MS/MS

Complete the form below to unlock access to ALL audio articles.
Dioxins, including polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), are a group of highly toxic organic compounds commonly formed as byproducts in waste combustion processes or from the industrial manufacture of chemicals such as chlorinated pesticides.
PCDDs and PCDFs pose a significant risk to human health and have been classified by the United States Environmental Protection Agency as carcinogenic even at trace levels. Consumption of contaminated food is a common route of dioxin exposure as these compounds enter, persist and bioaccumulate in the food chain. To ensure consumer safety, accurate detection and quantification of PCDDs and PCDFs in food and animal feed is essential.
Trace dioxin quantification by GC-MS/MS
Until 2014, European Union (EU) legislation required the confirmation and quantification of PCDDs and PCDFs in food and feed samples to be performed by gas chromatography-high resolution mass spectrometry (GC-HRMS). However, advances in the sensitivity and selectivity of gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS) systems have led to these criteria being updated. The EU now allows GC-MS/MS to be used for confirmatory analysis (as an alternative option to GC-HRMS) to help control maximum levels (MLs) and action levels of PCDDs and PCDFs in certain foodstuffs and animal feeds.
Key to this technological evolution has been the ability to overcome the challenge of confidently quantifying analytes in complex sample matrices. Currently, in GC-HRMS analyses, high mass resolution provides the high selectivity necessary for the separation of analytes of interest from interfering matrix components. In contrast, the latest GC-MS/MS platforms now utilize highly selective, compound-dependent precursor-to-product transitions to obtain confident detection and quantification, with little or no interference from other matrix components.
Low level quantification of PCDDs and PCDFs in food and animal feed
Given the importance of trace level dioxin analysis, analytical sensitivity has been a primary focus for both food safety laboratories and instrument providers. The latest GC-MS/MS technologies are enabling the determination of PCDDs at ultra-low levels, and recent studies highlight the exceptional performance of some of these systems.
The analysis of PCDDs and PCDFs in animal feedstuff samples was put to the test using a Thermo Scientific TSQ 9000 triple quadrupole GC-MS/MS system, equipped with an advanced electron ionization (AEI) source. The samples were prepared for analysis through a solvent extraction and cleanup step, which involved an offline multi-layered acidic silica column. The mass spectrometer was operated in selected reaction monitoring mode, with dwell time priority given to the specific transitions of native dioxin and furan congeners.
Instrumental detection limits (IDLs) are typically determined by two methods. One option is to calculate the signal-to-noise ratio of a peak, while the alternative is to determine IDL statistically using a series of repeat injections. The latter approach is generally adopted, as it involves the measurement of IDLs focused on a single compound using a limited number of transitions. However, since the analysis of PCDDs and PCDFs in feedstuff samples requires the use of several transitions for confirmation of both native and labeled congeners, and due to the very low levels used for these tests, it is more reasonable to define a method limit of quantitation (LOQ).
The EU mandates that the level of sensitivity must be equal to or less than one fifth of the ML for that specific food or feedstuff. Additionally, criteria around ion ratio tolerance (±15%), number of transitions (2), and quadrupole resolution (equal or better than unit mass) must also be met. So how does the GC-MS/MS system perform?
GC-MS/MS pushing the limits of quantitation
Table 1 shows the calculated LOQs for 17 dioxin congeners in an animal feed-grass quality control (QC) sample. The lowest calibration standard amount was adjusted for sample weight and toxicity equivalency factors (TEFs). These results highlight the exceptional sensitivity of the method, with the LOQ of the method being five times lower than that required by legislation. Essentially, this allows as little as 5 g of feed sample to be used, if required.
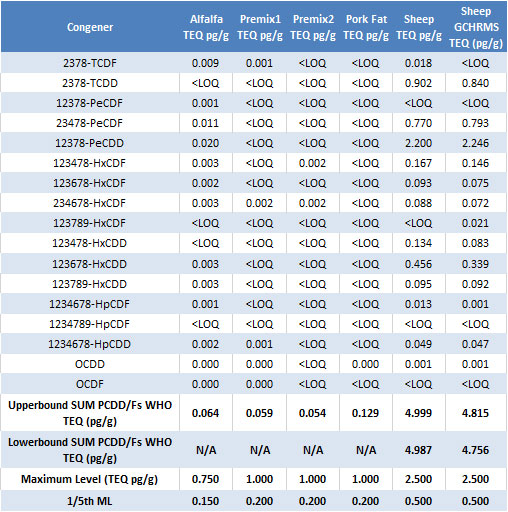
Regulatory criteria were met for all sample types analyzed and the results are shown in Table 2. The majority of the samples analyzed had very low levels of contamination present, as evidenced by the fact that many of the congeners were found to be below the limit of quantitation (<LOQ).
With human health in the balance, food safety laboratories rely on robust analytical methods to ensure that the products we put in our grocery basket meet regulatory standards. The results highlighted here demonstrate that the GC-MS/MS system configured with an AEI source is more than capable of quantifying dioxin congeners at low levels that go beyond the required LOQs. The instrument satisfies all of the current EU commission requirements for the detection and confirmation of dioxins in food and feed samples.
Table 1: Calculated LOQs for grass animal feed QC sample.