Exploiting the Antimicrobial Properties of Toadlet Peptide To Kill Bacteria
Complete the form below to unlock access to ALL audio articles.
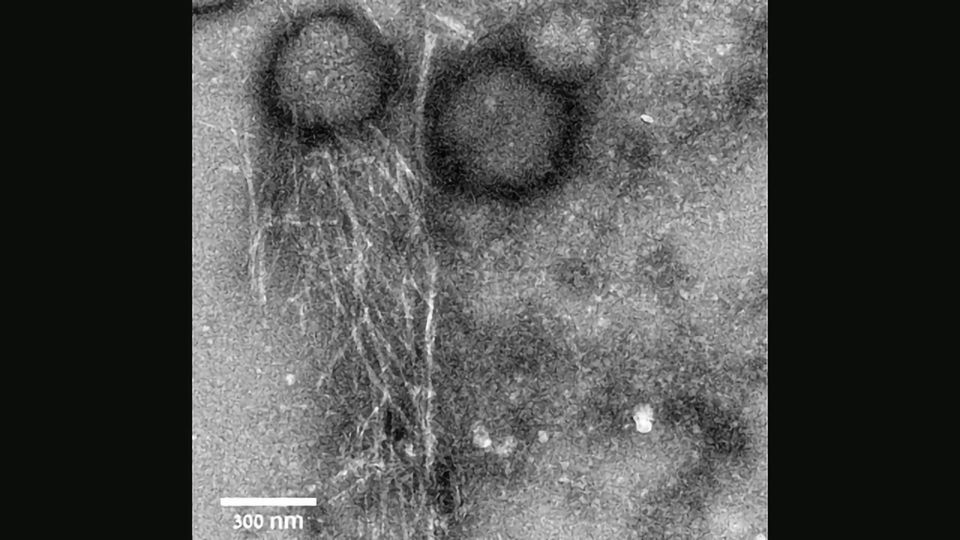
The researchers solved the 3D molecular structure of an antibacterial peptide named uperin 3.5, which is secreted on the skin of the Australian toadlet (Uperoleia mjobergii) as part of its immune system. They found that the peptide self-assembles into a unique fibrous structure, which via a sophisticated structural adaptation mechanism can change its form in the presence of bacteria to protect the toadlet from infections. This provides unique atomic-level evidence explaining a regulation mechanism of an antimicrobial peptide.
The antibacterial fibrils on the toadlet's skin have a structure that is reminiscent of amyloid fibrils, which are a hallmark of neurodegenerative diseases, such as Alzheimer's and Parkinson's. Although amyloid fibrils have been considered pathogenic for decades, it has recently been discovered that certain amyloid fibrils can benefit the organisms that produce them, from human to microbes. For example, certain bacteria produce such fibrils to fight human immune cells.
The findings suggest that the antibacterial peptide secreted on the toadlet's skin self-assembles into a "dormant" configuration in the form of highly stable amyloid fibrils, which scientists describe as a cross-β conformation. These fibrils serve as a reservoir of potential attacker molecules that can be activated when bacteria are present. Once the peptide encounters the bacterial membrane, it changes its molecular configuration to a less compact cross-α form, and transforms into a deadly weapon. "This is a sophisticated protective mechanism of the toadlet, induced by the attacking bacteria themselves," says structural biologist Meytal Landau, the lead author of this study. "This is a unique example of an evolutionary design of switchable supramolecular structures to control activity."
Potential for future medical applications
Antimicrobial peptides are found in all kingdoms of life, and thus are hypothesised to be commonly used as weapons in nature, occasionally effective in killing not only bacteria, but also cancer cells. Moreover, the unique amyloid-like properties of the toadlet's antibacterial peptide, discovered in this study, shed light on potential physiological properties of amyloid fibrils associated with neurodegenerative and systemic disorders.
The researchers hope that their discovery will lead to medical and technological applications, e.g. development of synthetic antimicrobial peptides that would be activated only in the presence of bacteria. Synthetic peptides of this kind could also serve as a stable coating for medical devices or implants, or even in industrial equipment that requires sterile conditions.
Reference: Salinas N, Tayeb-Fligelman E, Sammito MD, et al. The amphibian antimicrobial peptide uperin 3.5 is a cross-α/cross-β chameleon functional amyloid. PNAS. 2021;118(3). doi:10.1073/pnas.2014442118
This article has been republished from the following materials. Note: material may have been edited for length and content. For further information, please contact the cited source.

