High Throughput Mass Spectrometry-Based Screening Assay Trends

Complete the form below to unlock access to ALL audio articles.
Introduction
Mass spectrometry (MS) is a data rich, label-free free assay format with broad applicability to phenotypic analyses. By monitoring changes in the mass to charge ratio of molecular ions it enables the direct measurement of substrate to product conversion in a reaction. This is accomplished without the involvement of any labelling or specific assay reagent technologies, and if the throughput were available would represent a highly attractive generic low cost screening technology. Unfortunately conventional MS typically involves a liquid chromatography (LC) step to remove non-volatile salts and buffer components that can suppress ion formation. This limits the throughput to between 1 and 15 minutes per sample, which severely restricts the application of conventional MS systems, and precludes it from most screening-related activities.
The current state-of-the-art with respect to higher throughput MS consists of relatively few options. The most well-known of these is Agilent RapidFire 365 which is a fully integrated MS system that delivers automated high-capacity sample analysis. It uses an injection-based MS and involves ONline sample prep. It offers cycle times as fast as 8 sec/sample or just under 1hour/384 plate processed. Alternative approaches include gaining additional throughput and capacity by operating multiple LC-MS systems in parallel; ONline multiplexing up to 4 parallel LC systems to a single MS (e.g. Thermo Scientific Transcend System); and utilizing strategies like substrate multiplexing and/or compound pooling to enable simultaneous testing of more targets or more compounds. Other MS methodologies claiming higher throughput include LAESI-MS imaging that oblates samples direct from microplates wells or slides with minimal sample prep prior to analysis (e.g. Protea LAES DP-1000 System) and surface-based MALDI-MS involving acoustic sample deposition of complex biologically mixtures to an arrayed substrate chip without prior sample prep (e.g. NextVal MassInsight). Finally emerging techniques for the direct loading of samples into MS systems (e.g. acoustic dispensing using Labcyte Echo) may offer the greatest promise in enabling high throughput, low cost, label-free analysis.
In November 2014 HTStec undertook a market survey on high throughput MS-based screening assays mainly among research labs in pharma, biotech and academia. The survey was initiated by HTStec as part of its tracking of emerging life science marketplaces. The objectives were to comprehensively document the current approaches to implementation and use of HT MS-based screening assays, and to understand future user requirements/preferences. This article contains ‘selected findings’ from the HTStec market report, High Throughput Mass Spec-Based Screening Assay Trends 2014. It is intended to provide the reader with a brief insight into recent market trends. It covers only 10 out of the 30 original questions detailed in the full report. The full published report should be consulted to view the entire dataset, details of the breakdown of the responses for each question, its segmentation and the estimates for the future. Please click here for more information.
Why use MS-based screening assays:
The most compelling reasons for wanting to use MS-based screening assays are reported in Figure 1. Able to quantify multiple analytes simultaneously in single sample was ranked the most compelling reason for using MS-based screening assays. This was closely followed by quantitation of direct endpoints, avoiding use of coupled assays; true label-free detection technology; and then avoids molecular labels, fluorescent dyes, reporters, antibodies etc. Ranked least compelling amenable to endogenous endpoint quantification from native single cells.
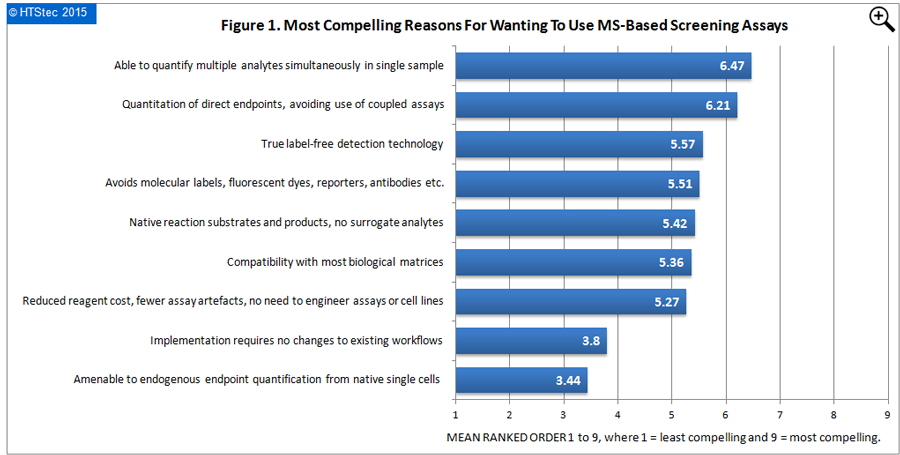
Figure 1. Most Compelling Reasons For Wanting To Use MS-Based Screening Assays
What respondents most want to analyze using MS-based screening assays:
The materials survey respondents were mainly interested in analyzing by MS-based screening assays are presented in Figure 2. This showed that substrates/metabolites/reaction products was ranked highest (most likely to analyze). This was closely followed by small molecules; peptides; proteins; and then fragments.

Figure 2. What Respondents Most Want To Analyze Using MS-Based Screening Assays
Target classes where MS-based screening approaches have application:
The target classes where survey respondents are most interested in applying MS-based screening approaches are reported in Figure 3. This showed that kinases was the target class where there was greatest interest in applying MS-based screening approaches. This was followed by ADME-Tox & PK; epigenetic targets and then other enzymes. Least interest was for other cytoplasmic receptors.

Figure 3. Target Classes Respondents Are Most Interested in Applying MS-Based Screening Approaches
Biological sample types to be analyzed by MS-based screening approaches:
The types of biological samples to be analysed by MS-based screening approaches are given in Figure 4. Soluble aliquots taken from homogeneous assays were rated the biological samples most likely to be investigated. This was followed by lysates from cell-based assays; biological fluids collected from in vivo studies; and then intact individual cells or cell layers. Least interest was in 2D tissues sections and intact 3D cellular microtissues.

Figure 4. Types Of Biological Samples Most Interested To Analyse By MS-Based Screening Approaches
ADME applications of MS-based screening:
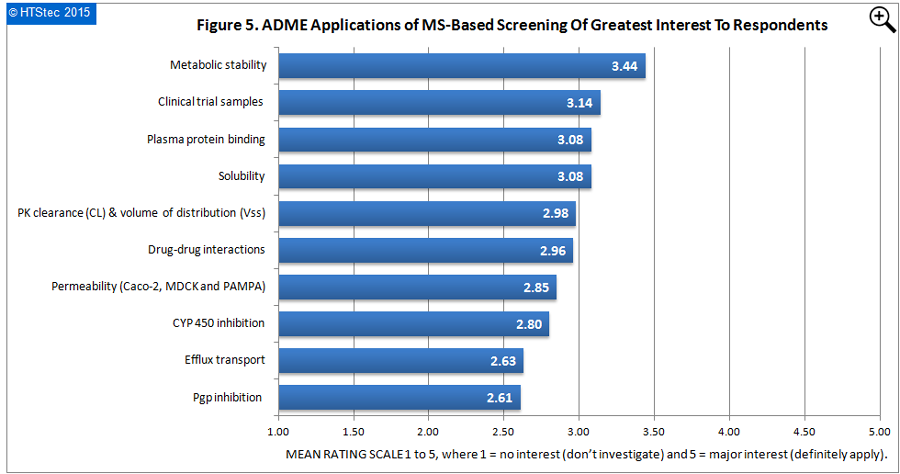
The ADME applications of MS-based screening of greatest interest to survey respondents are presented in Figure 5. This showed metabolic stability was rated the ADME area where there was greatest interest. This was followed by clinical trial samples, and then plasma protein binding and solubility. Least interest was for Pgp inhibition.
Main limitations of MS-based screening:
The extent to which potential issues associated with MS-based screening assays are limiting is presented in Figure 6. This showed high cost of instrumentation was ranked the main limitation associated with MS-based screening assays today. This was followed by lack of fully-automated truly high throughput systems; requires an experienced operator; and then need for sample prep prior to analysis. Least limiting was sample volume too large for miniaturized screening.

Figure 6. Extent To Which Potential Issues Associated With MS-Based Screening Assays Are Limiting
Feedback on what is implied by high throughput MS-based assays:
The survey found that a median of at least 10K-25K samples processed per 24 hour day constituted true high throughput screening (HTS) to Pharma respondents. They also desired a median MS-based assay throughput of 5 times that achievable with the Agilent Rapidfire RF365 today (i.e. 5 times a 384 plate/hour). Survey respondents views on the future adoption and deployment of available MS-technology platforms was that compatibility with 1536 plates and low volume sample utilization (<5uL) were both nice to have options that would influence future purchasing. To achieve higher throughput MS-based primary screening the majority of respondents were also prepared to consider compound pooling (drug mixtures) and multiplexing strategies (e.g. multiplexing different targets in the same assay; multiplexing the same target using different peptide substrates in the same assay; or multiplexing multiple LC systems to a single MS).
Where high throughout MS-based screening assays are expected to be most used:
Where respondents plan (or make) most use of high throughput MS-based screening assays is reported in Figure 7. This showed that high throughput MS-based screening assays are expected to make the biggest impact in the future (2016) on primary screening (HTS). This was followed by profiling/secondary/ orthogonal/counter screening; and then mode of action studies and ADMET & PK assays.
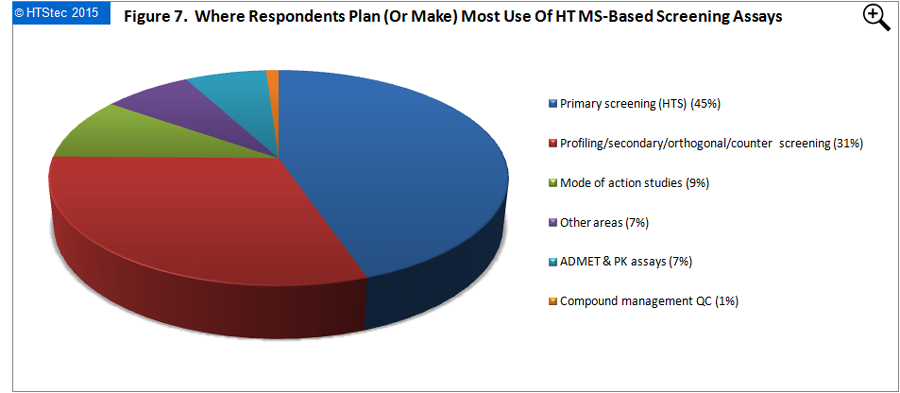
Figure 7. Where Respondents Plan (Or Make) Most Use Of HT MS-Based Screening Assays
Most preferred approach to truly high throughput automated MS-based screening assays:
The approach to enabling truly high throughput automated MS-based screening assays expected to gain most traction in the future is reported in Figure 8. This showed that injection-based MS that typically involves ONline sample prep (e.g. Agilent RapidFire) (43% selecting) was the most preferred approach. This was followed by acoustic dispensing directly into MS (e.g. using Labcyte Echo) and substrate multiplexing and/or compound pooling combined with injection-based MS that typically involves ONline sample prep (both 17% selecting). Least traction was expected in surface-based MALDI-MS involving acoustic sample deposition of complex biologically mixtures to an arrayed substrate chip without prior sample prep (e.g. NextVal MassInsight).
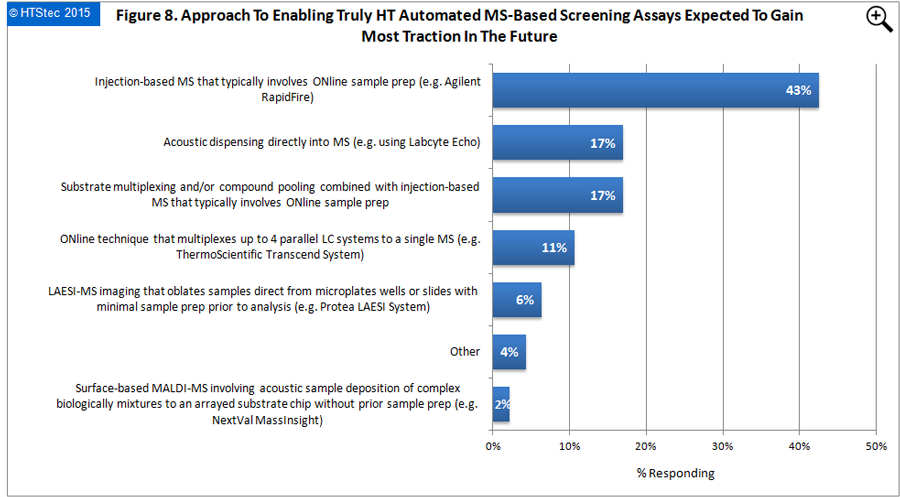
Figure 8. Approach To Enabling Truly HT Automated MS-Based Screening Assays Expected To Gain Most Traction In The Future
Preferred vendors of MS detection systems:
The preferred vendors for MS detection systems which survey respondents would seek to invest in if new high throughput approaches to MS-based screening assays were available are given in Figure 9. The preferred vendors of MS detection systems were Sciex (28% share); Agilent (21% share); Thermo Scientific (20% share); and Waters (14% share). All other vendors had less than 10% share.

Figure 9. Preferred Vendors Of New MS Detection Systems Purchases. PLEASE NOTE this vendor preference should not be regarded as a true market share. It is based on survey respondent's preferred vendor if purchasing a new MS detection system, not on the total $ value of actual sales.
Respondents views on the status of MS-based screening assays:
The level of agreement of survey respondents with some statements about the status of MS-based screening assays are presented in Figure 10. Agreement with the statements was most positive (greatest agreement) with ‘enabling truly high throughput MS-based HTS will have far-reaching implications’; and most negative (greatest disagreement) with ‘MS-based assays will never be the preferred screening format for all our assays’. The statement which was least controversial (i.e. respondent neither agreed nor disagreed) was ‘MS-based assays are cheaper than other assay formats to implement’.
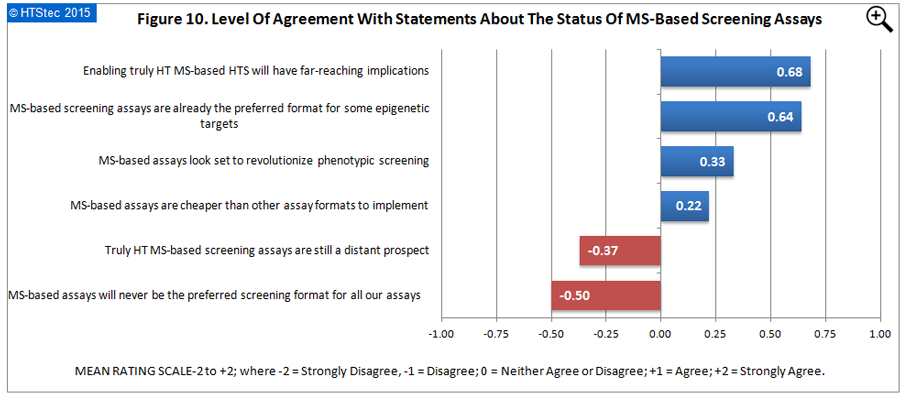
Figure 10. Level Of Agreement With Statements About The Status Of MS-Based Screening Assays
Conclusions:
The selected findings reported above revealed that the ability to quantify multiple analytes simultaneously in a single sample was the most compelling reason for wanting to access MS-based screening assays. With MS being a true label-free technology approach ranked not far behind. With respect to MS-based screening assays survey respondents mainly want to be able to analyze substrates/metabolites/reaction products. Current interest is not surprisingly focused in applying MS-based screening approaches to enzyme targets like kinases and epigenetic proteins with soluble aliquots taken from homogeneous (mainly biochemical) assays most investigated. Although the potential to extend these studies to lysates from cell-based assays is greatly desired. Interest is also high in the ADME application area with metabolic stability assays currently most appealing from an MS assays perspective. The significant cost of MS instrumentation, followed by the lack of fully-automated truly high throughput MS systems remain the biggest factors limiting the adoption of MS-based screening assays today. Although some higher throughput MS systems are available, the throughput achievable from the best of these systems (e.g. Agilent Rapidfire RF365 system) currently is at least 5 times less than that required to significantly impact primary screening (HTS) and enable screening of large libraries. Despite this lack of desired throughput the current survey respondent’s preferred approach to achieve high throughput remains with what is currently commercially available i.e. injection-based MS that typically involves ONline sample prep (e.g. Agilent RapidFire). The next approach which expected to gain most traction in the future is the emerging concept of acoustic dispensing directly into MS (e.g. using Labcyte Echo).
The future of high throughput MS-based screening assays is now at a tipping point following a presentation at the SLAS Meeting at Washington, DC in February 2015 by scientists from Astrazeneca, Labcyte and Waters who have successfully utilized acoustic droplet ejection like a standard MS electrospray injection. This development has the potential to foreshadow existing MS approaches that rely on liquid chromatography separations. Integration of acoustic droplet ejection and MS could potentially result in a system capable of delivering around 4,000 data points per hour into a high-sensitivity label-free detector. Several instrument configurations are under investigation and an optimized high throughput product has yet to come to the marketplace, but the ability to load samples from 1536 plates into a mass spectrometry detector, without LC sample prep, at a high rate from minimal assay volumes (as low as several µLs) has potential to significantly impact drug discovery, but also to influence research in other life science areas.
Survey respondent’s views on some statements about the future status of MS-based screening assays are therefore highly appropriate in the circumstances. Their high level of agreement with the statement ‘enabling truly high throughput MS-based HTS will have far-reaching implications’ and strong disagreement with the statement ‘MS-based assays will never be the preferred screening format for all our assays’ are both consistent with a future where high throughput MS-based instrumentation truly amenable to the needs of screening labs are commonplace.
Author: John Comley, Managing Director, HTStec Ltd.
DISCLAIMER: HTStec Limited has exercised due care in compiling and preparing these Selected Findings from its Report, which is based on information submitted by individuals in respondent companies. HTStec Limited has NOT verified the accuracy of this information, nor has it is established respondent’s authority to disclose information to HTStec Limited. HTStec Limited expressly disclaims any and all warranties concerning these Selected Findings including any warranties of merchantability and/or fitness for any particular purpose, and warranties of performance, and any warranty that might otherwise arise from course of dealing or usage of trade. No warranty is either expressed or implied with respect to the use of these Selected Findings. Under no circumstances shall HTStec Limited be liable for incidental, special, indirect, direct or consequential damages or loss of profits, interruption of business, or related expenses that may arise from use of these Selected Findings, including but not limited to those resulting from inaccuracy of the data therein.

