Rebalancing RAS To Treat COVID-19-induced Lung Inflammation

Complete the form below to unlock access to ALL audio articles.
Technology Networks recently had the pleasure of speaking with Rohit Batta, Chief Medical Officer at Vicore to learn more about “VP01”– a novel, first-in-class, orally available drug in development for interstitial lung diseases, including COVID-19.
Batta elaborates on VP01’s mechanism of action and provides further insight into the ATTRACT study, which was recently launched in response to the urgent need to discover effective treatments for COVID-19. We also discuss how the COVID-19 pandemic is impacting drug development pipelines.
Laura Lansdowne (LL): Could you tell us more about Vicore Pharma, your mission, and area of expertise?
Rohit Batta (RB): Vicore Pharma is an innovative international research and development pharmaceutical company dedicated to creating life-changing treatments for lung conditions, with a focus on rare diseases. Embedded in our approach is the determination to find solutions for conditions such as COVID-19, which can have an enormous impact on patients and their families, aiming to transform the lives of those affected.
LL: How has the COVID-19 pandemic impacted your pipeline and existing clinical development programs?
RB: Like all industries, the pharmaceutical sector has had to adapt to how it operates in light of COVID-19 – perhaps more swiftly than others given its importance in treating patients. As the pandemic progressed, we began to see patient enrolment in our Raynaud Phenomenon mechanistic trial slow and eventually, stop as facilities moved to prioritize patient safety and prepare themselves for an influx of COVID-19 cases. Sites were justifiably prioritizing care over clinical research activities. Alongside this, some of the UK transport restrictions set up to stop and slow the spread of the virus prevented in-person site visits.
In parallel, we were confident that repurposing our existing medicine, VP01, could have a real chance in turning around COVID-19 with respect to the hyperactive immune system, and easing pressure on hospitals, so we swiftly started to plan our Phase II clinical study. The regulators matched us by approving the study in record time (four weeks). The Medicines and Healthcare products Regulatory Agency (MHRA) were incredibly responsive during that time, especially as many were working from home – to give an example, we requested scientific advice and were granted a meeting the next day! To provide some context, these study approvals, particularly for drugs targeting more common diseases, can take several months to reach approval.
Now, in some territories, we are beginning to see more movement and freedom. In the UK, for example, we are now seeing movement away from the lockdown, which will hopefully enable some studies to open up for recruitment. This is positive but it is critical that it is done in such a way that adapts to the environment to maintain patient safety.
LL: What is VP01, could you elaborate on its mechanism of action and the underlying basis for exploring it as a COVID-19 treatment?
RB: VP01, is a potent and selective angiotensin II (Ang II) type 2 receptor (AT2R) agonist. It is a novel, first-in-class, orally available medicine in development for interstitial lung diseases, including idiopathic pulmonary fibrosis (IPF) and now COVID-19.
When COVID-19 enters the human body, it uses an enzyme called ACE2 as a gateway and disrupts the enzyme’s usual role of balancing two opposing pathways in what’s called the renin–angiotensin system (RAS).
This causes a major RAS imbalance which can lead to acute inflammation and can contribute to the creation of a ‘cytokine storm’ in the lungs where the body’s own immune system overreacts and attacks itself.
VP01 rebalances the RAS by activating its protective pathway, which has the potential to help bring the body back to normal function
LL: LifeArc recently awarded you a grant of £ 1.5 million for the VP01 COVID-19 clinical study, could you tell us more about the study?
RB: The lack of any treatments has created a need of unprecedented urgency so we have been working tirelessly to start the VP01 ATTRACT study (Angiotensin II Type Two Receptor Agonist COVID-19 Trial). Given this pressing need for effective medicines for COVID-19, we are conducting a randomized, double-blind, placebo-controlled Phase II trial evaluating the safety and efficacy of VP01 in patients with COVID-19 infection who have been hospitalized and are not requiring mechanical invasive or non-invasive ventilation. These patients can have an early intense inflammatory drive in the lungs, which could lead to acute respiratory failure if it progresses.
The aim is to intervene early in order to prevent more serious complications such as pneumonia and/or mechanical ventilation resulting from a hyperactive immune system.
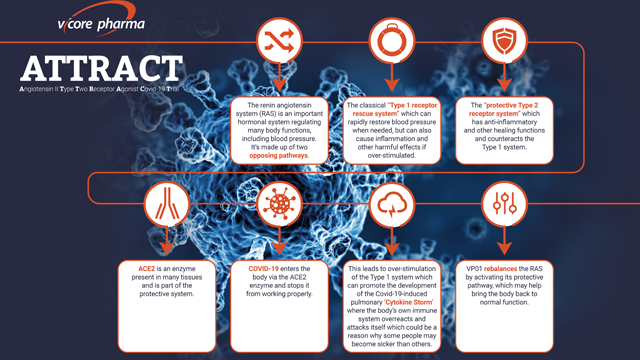 An overview of the ATTRACT trial. Credit: Vicore.
An overview of the ATTRACT trial. Credit: Vicore.
Approximately 100 subjects will be randomized in a 1:1 schedule to receive either standard of care plus placebo or standard of care plus VP01 for seven days and then seven days follow up. Data from the trial will inform and guide the design of further clinical investigations of VP01 for COVID-19.
It is challenging to comment on timelines as we need to have the flexibility to adapt to the situation as it develops, but we are working on the assumption that we will have topline results by Q4 2020.
LL: It must be challenging to continue this vitally important work amidst the pandemic, what approaches have you and your team taken to adopt the “new normal”?
RB: Vicore Pharma is an international business with headquarters in Sweden but bases across the globe, so creating virtual teams working many hundreds of miles away from each other has been sort of the norm. Our ethos has been not necessarily “work from home”, but “work from anywhere” that motivates and inspires us. That, mixed with the Swedish work ethics of being respectful and doing what is commonly deemed to be "the right thing to do" has enabled us to easily adapt to a remote way of working and to shift our research focus from primarily rare lung disorders to COVID-19.
On a personal level, homeschooling nine-year-old twins has been another aspect to lockdown, where on some days I’ve ended up as a professional entertainer. It’s been fun teaching them about menacing viruses, but most of their lockdown education has come via virtual lessons from school, pushing our broadband connection to the limit!
Rohit Batta was speaking with Laura Elizabeth Lansdowne, Senior Science Writer for Technology Networks.


