Using Bioprinted Tissue Models To Test Drugs Against Diseases Including COVID-19

Complete the form below to unlock access to ALL audio articles.
Technology Networks recently had the pleasure of speaking with Keith Murphy, CEO and founder of Viscient Biosciences to learn how the company is leveraging its expertise in 3D disease models and multi-omics approaches to drive drug discovery efforts.
In addition to their ongoing work using 3D bioprinting and tissue engineering techniques to uncover novel drug targets for fatty liver disease, Keith also highlights their efforts to test drugs against the novel coronavirus (SARS-CoV-2) using bioprinted lung tissue.
Laura Lansdowne (LL): How have advances in disease modeling impacted drug discovery success?
Keith Murphy (KM): Disease modeling is essential to drug discovery. The ability to use disease models in the lab has propelled the discovery and development of new and better medicines to treat disease. However, not all disease models are created equal. Animal models and cells grown individually in 2D (cell culture) have numerous drawbacks and limitations, and in many cases, cannot provide an accurate model of disease. This is because of differences between animal and human biology and many types of cells in 2D without normal cell–cell interactions stop behaving as they do in the body. For diseases where multiple cell types interact, such as in fibrotic or inflammatory diseases, we have used 3D bioprinting and tissue engineering to uncover novel drug targets. We have done this in fatty liver disease (nonalcoholic fatty liver disease (NAFLD)/ nonalcoholic steatohepatitis (NASH)), the most common chronic liver condition (~1 in 4 adults in the U.S.), and are now working on lung tissue to test drugs to treat SARS-CoV-2, the coronavirus that causes COVID-19.
LL: How are you leveraging your expertise in 3D disease models and multi-omics to drive drug discovery?
KM: At Viscient Biosciences, we follow the principle “take the disease out of the patient and put it in a dish.” We start with human cells from patients who have the disease. We then use technologies of 3D bioprinting and tissue engineering combined with multi-omics, including single-cell genomics, to develop the best possible picture of that disease. We get more than 64 million data points on each tissue per timepoint to know which genes are turned on/off inside not only the whole tissue, but also specific cells. Viscient’s disease models provide high-quality information to uncover novel biology, identify new drug targets, and test disease-modifying drugs. For NAFLD/NASH, we have modeled disease progression in vitro, identified novel disease-driver gene targets, and are advancing medicinal chemistry to develop drugs that modulate these targets.
LL: What are the key benefits of combining organoid technology with bioprinting and single-cell analysis?
KM: At Viscient, we are focused on starting with the most highly accurate representation of human disease early in the drug discovery process. Classical drug discovery often starts with an animal model, and then moves to cell-based screening to find promising drug chemistry candidates from hundreds of thousands of chemical compounds. Clinical trial failures have been attributed to heavy reliance on imperfect animal models of disease and 2D cell-based screening in classical drug discovery. Drug discovery at Viscient replaces animal models with our bioprinted disease models, which provide a better, more accurate disease model to identify drug targets, especially for more complex diseases. We then screen drug candidates in organoids, which are a simpler model than bioprinted tissues, but they still provide 3D- and multi-cell interactions. The best candidates are then put into the bioprinted model for selection and optimization of a lead drug. This allows us and our partners to have the best of both worlds and have more accurate models early in drug discovery, with the goal of avoiding clinical failures later down the line.
LL: Could you touch on the importance of and challenges related to developing drugs for liver disease?
KM: There are two major problems in discovering and developing drugs for liver disease. The first is that animal models are poor predictors of a drug’s success in treating the disease in a human – success in animal models does not “translate” to success in clinical trials. Dozens of drugs have failed in clinical trials in NASH and liver fibrosis. The second is that, because animal models rarely translate to clinical success, they also cannot be used to find new drug targets in liver disease. There are currently no FDA approved drugs to treat NASH. That’s why new disease models, like Viscient’s 3D NASH model, are essential. In fact, we have identified a set of novel targets that can alter disease progression in our NASH model, and we are now exploring these novel targets to identify disease-modifying drug candidates.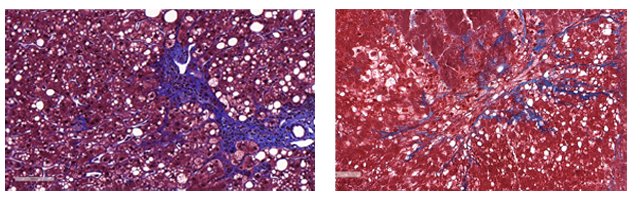 Liver biopsy from a patient with NASH (left). Viscient’s bioprinted NASH model (right). Fatty droplets (white spaces in the tissue) and collagen fibers (stained blue) are seen in both the patient biopsy and Viscient’s bioprinted tissue. In NASH, fat builds up in the liver and collagen is a marker of fibrosis.
Liver biopsy from a patient with NASH (left). Viscient’s bioprinted NASH model (right). Fatty droplets (white spaces in the tissue) and collagen fibers (stained blue) are seen in both the patient biopsy and Viscient’s bioprinted tissue. In NASH, fat builds up in the liver and collagen is a marker of fibrosis.
LL: Could you tell us more about your work related to NASH and coronavirus (COVID-19)?
KM: The most impressive thing about Viscient’s bioprinted NASH model is how well it recapitulates the disease in humans. We have queried the gene expression profile (which genes are turned on/off in disease) of our bioprinted NASH model and the profile matches that of liver biopsies of NASH patients. The patient biopsies and our bioprinted tissues even look the same under the microscope (see image). With Viscient’s bioprinted NASH model, we have identified a novel biological pathway that is a major contributor to disease progression. When we looked in animal models, the pathway is different, providing further validation to our core hypothesis that 3D bioprinting creates more accurate models of human disease.
For COVID-19, we are creating a bioprinted lung tissue to test potential COVID-19 therapies to accelerate development. Previous research has already shown that 3D human lung tissue better models viral infectivity compared to regular cell culture. Viscient has an opportunity to help speed potential therapies against the novel coronavirus to patients, and we are moving quickly to do that.
Keith Murphy was speaking with Laura Elizabeth Lansdowne, Senior Science Writer for Technology Networks.


