Why Should Antibodies Be Manufactured Recombinantly?

Complete the form below to unlock access to ALL audio articles.
Given that bad antibodies are likely to blame for a significant proportion of the reproducibility crisis in science, any efforts to reduce batch-to-batch variability are more than welcome.
One company with a plan to help in this area is “Absolute Antibody”, who believe that recombinant antibodies are the way forward. To learn more about making antibodies with recombinant technology, we spoke to Ian Wilkinson, PhD, Chief Scientific Officer.
Michele Wilson (MW): What are recombinant antibodies? How do they differ from monoclonal antibodies?
Ian Wilkinson (IW): In theory monoclonal antibodies bind to a single epitope on an antigen, meaning that all antibodies within the sample are the same (unlike a polyclonal). Traditionally, monoclonal antibodies have been produced from fusions of an antibody-producing B-cell from an immunised animal with a myeloma cell. This creates an immortal antibody-producing cell known as a hybridoma. The vast majority of reagent antibodies are produced from hybridomas. Hybridomas suffer from many limitations: they can undergo genetic drift leading to batch-to-batch variability; they can be genetically unstable and stop expressing the antibody; and about a third of hybridomas are not actually monoclonal and contain additional antibody genes (see this paper, and our blog post here).
Although a hybridoma-derived monoclonal antibody is characterized for function, the exact sequence of the antibody is unknown. A recombinant antibody is a type of monoclonal antibody where the sequence has been identified and then produced synthetically; for example, in human embryonic kidney (HEK) or Chinese Hamster Ovary (CHO) cells. As recombinant antibodies are defined at the sequence level, you can guarantee production of a truly monoclonal antibody with no batch-to-batch variation. On top of this, having access to the sequence opens up a myriad of antibody engineering opportunities (which will be discussed in later questions).
MW: Can you give us an overview of the main steps? How are antibody genes recovered, amplified and cloned?
IW: Obtaining an antibody sequence from a hybridoma cell line usually involves identifying and amplifying antibody-coding mRNA transcripts from the cells, which can be done using primers to amplify the genetic sequence of the antibody variable domains (also called ‘binding domains’ or ‘V-region’). The two main approaches to achieve this are known as V-region PCR and 5’ RACE. These approaches are limited in that they are low-throughput, offer no quantification, are often slowed by extra antibody chains and other complications, and use predefined primers that might introduce artifacts into the antibody sequence.
At Absolute Antibody, we do things slightly differently. We use random primers to sequence the whole mRNA transcriptome by high-throughput Next Generation Sequencing (NGS) and have developed a proprietary software algorithm to analyze the vast amount of sequencing data and extract the antibody sequence. From there the antibody genes can be synthesized and cloned into expression vectors using standard molecular biology techniques. This method can be applied to any antibody species and isotype, returns all of the antibody sequences that are present in a hybridoma along with their relative abundance, and allows us to process 50+ hybridomas at once.
MW: What are the benefits of using recombinant antibodies?
IW: All recombinant antibody production starts with a DNA sequence, meaning that a defined antibody will be produced, at a higher purity than traditional monoclonals, and with minimal batch-to-batch variation. In the last few years, reproducibility in life sciences research has been a growing concern, and poorly characterized antibodies have been pointed out as culprits. (See also: a 2015 Nature article signed by over 110 scientists calling for antibody standardization across all life sciences research).
Using recombinant antibodies means that researchers can reference reagents that are absolutely defined at the sequence level, offering the highest level of reproducibility for another scientist to replicate and build on a particular result.
In addition, with a sequence available, engineers such as Absolute Antibody can go a step further and start to customize antibodies to better suit different applications. Options become available to researchers that wouldn’t be possible with a traditional monoclonal. Recombinant antibody technology has been around since the eighties, but the technology was mainly restricted to the pharmaceutical industry until Absolute Antibody was founded in 2012 with a view to bring engineered antibodies to the wider life sciences community.
MW: Once you have the variable domain sequences of your antibody, what engineering opportunities can be applied?
IW: Engineering lets us easily switch antibody species and format, which gives researchers more options when considering reagents for their experiments, or match antibody to host organism for reduced immunogenicity in in vivo studies. Each recombinant antibody in Absolute Antibody’s catalog is available in a range of species and formats.
Going further, we can engineer and produce non-IgG antibody isotypes such as IgE and IgM for use as calibrators and controls. Or, we can introduce mutations into the antibody constant region to remove Fc receptor (FcγR) binding to abrogate ADCC in vivo or reduce background staining in flow cytometry. We can also make antibody fragments, or combine multiple antibody specificities into bispecific and trispecific antibodies - whatever the application demands. The number of antibody formats we can engineer is almost unlimited.
Some examples of antibody engineering are summarized in the below diagram:
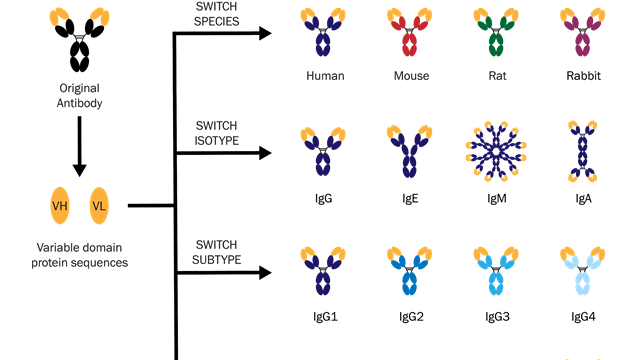
This diagram shows how switching variable domains (VH or VL) can create antibodies that bind to antigens from different species, or exist as different isotypes or subtypes. Image credit: Absolute Antibody
MW: At present, roughly what proportion of antibodies used in research are recombinant antibodies? Do you expect this to change? If so, how?
IW: It’s difficult to obtain an accurate number for this. We believe there are at least 700,000 monoclonal antibodies available in the reagents market. Analysis of known suppliers of recombinant antibodies that clearly identify those antibodies as recombinant suggests that at most about 3% of antibodies available to researchers are manufactured recombinantly. Large antibody suppliers are beginning to offer a recombinant range within their product offerings, but we are still the only antibody company that is solely recombinant.
The demand for recombinant antibodies is rapidly increasing, however, due to their lack of batch-to-batch variability and ability to open up engineering possibilities. We therefore expect the growing trend toward using recombinant antibodies to continue.
MW: What are you most excited about, with regards to antibody engineering?
IW: Bispecific antibodies have really taken off in the clinic, with preclinical and clinical data showing potencies that far surpass those achieved with monospecific or combination antibody therapies. At Absolute Antibody, we’ve made bispecific antibodies available to the wider research community with our range of bispecific reagents tailored for immunotherapy research in mice. This was a world-first for the reagents market, and enables researchers and drug developers alike to more easily evaluate potential bispecific combinations in mouse models.
We’re also excited about using engineering to improve antibodies’ in vivo performance, in particular by matching antibody species to the host organism for reduced immunogenicity. For example, we recently showed that a species-matched engineered antibody was able to deplete CD8+ T-cells in mice more completely and for longer than the traditional rat monoclonal antibody. By demonstrating these new options and making them readily available, we hope that the creativity of the scientific community will help us drive the next big advances in engineered antibody reagents.
Ian Wilkinson was speaking with Michele Wilson, Science Writer for Technology Networks.


