Latest Posters

Poster
Addressing the challenges of poor solubility: Rapid development and clinical evaluation of a lipid based formulation to enhance oral bioavailability of amuvatinib (MP-470)
Physiochemical and biopharmaceutical properties of new chemical entities are presenting increasing challenges to successful oral drug delivery. Here we present data on amuvatinib, a novel multi-targeted tyrosine kinase inhibitor specifically designed to be a potent inhibitor of mutant c-Kit and PDGFRalpha.

Poster
Quality Standards for 14C API for use in human clinical studies
The Good Manufacturing Practice (GMP) state that the active pharmaceutical ingredient (API) intended for use in early stage clinical trials should be of "suitable" quality. The Clinic Ready quality standard ensures that the API is synthesised with all the appropriate documentation to facilitate QP release of the final IMP for guman clinical dosing.

Poster
Elucidation of the Relative Bioavailability of a Drug Candidate from Different Regions of the Human Gastrointestinal Tract
This poster describes a pharmacokinetic study to investigate the relative absorption of an NCE from different regions of the human gastrointestinal tract, to support potential development of a sustained-release formulation.

Poster
Evaluation Of Single Point And IC50 Shift Assays For Measuring Time-Dependent Inhibition Of Drug Discovery Compounds
The aim of this study is to evaluate different assay designs, and data analysis methodology for measuring the extent of TDI for known inhibitors. We propose a reversible inhibition and TDI screening platform to cover early phase compounds, which enables accurate decisions to be made regarding development of compounds which could cause DDIs.
Poster
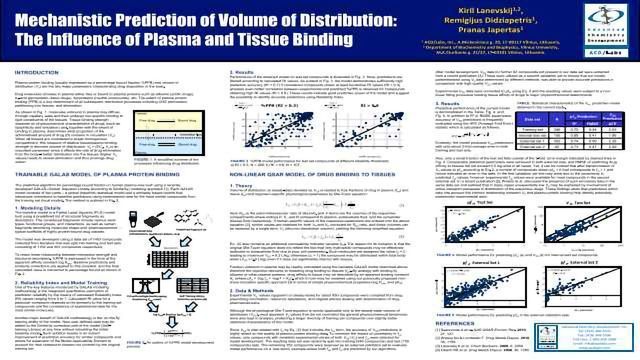
Mechanistic Prediction of Volume of Distribution: The Influence of Plasma and Tissue Binding
Plasma protein binding (usually expressed as a percentage bound fraction %PPB) and volume of distribution (Vd) are the two major parameters characterizing drug disposition in the body.

Poster
Probabilistic Predictive Model of the Human Liver Microsomal Metabolism Regioselectivity
Analytical identification of metabolites for a drug candidate is usually a time consuming and low-throughput task which is performed only in late drug development phases.Therefore, the ability to predict possible sites of human liver microsomal metabolism using in silico techniques would be highly beneficial for any medicinal chemist.

Poster
GALAS Modeling Methodology Applications in the Prediction of the Drug Safety Related Properties
Early computational evaluation of drug candidate properties related to its pharmaceutical safety (such as hERG inhibition induced cardiotoxicity or CYP3A4 inhibition responsible various unwanted drug-drug interactions) is becoming increasingly important in the drug discovery process.
Poster
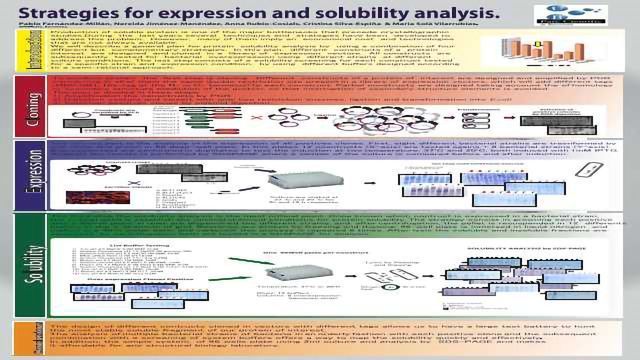
Strategies for expression and solubility analysis
Production of soluble protein is one of the major bottlenecks that precede crystallographic studies.During the last years several techniques and strategies have been developed to address this problem. However, many of them imply an economical cost and technologies that are not always available. We will describe a general plan for protein solubility analysis by using a combination of four different but complementary strategies. In this plan, different constructs of a protein interest are designed
Poster
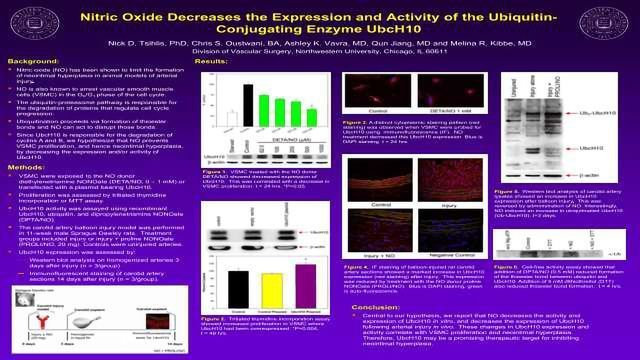
Nitric Oxide Decreases the Expression and Activity of the Ubiquitin-Conjugating Enzyme UbcH10
Nitric oxide (NO) has been shown to limit the formation of neointimal hyperplasia in animal models of arterial injury. Ubiquitination proceeds via formation of thioester bonds and NO can act to disrupt those bonds. We report that NO decreases the activity and expression of UbcH10 in vitro, and decreases the expression of UbcH10 following arterial injury in vivo. Therefore, UbcH10 may be a promising therapeutic target for inhibiting neointimal hyperplasia.

Poster
SuperNatural: A Database of Available Natural Compounds
The majority of marketed drugs are natural compounds or derivatives thereof. The compounds availability is often unclear. Therefore we have compiled a database of ~50,000 natural compounds. Starting point for in silico screenings are about 2,500 well-known, classified natural compounds or imported molecules. Possible medical applications can be detected and about three million conformers computed to account for the flexibility during usage of the 3D-superposition algorithm.
Advertisement

