224-year-old Smallpox Treatment Modified for COVID-19 Candidate Vaccine
Complete the form below to unlock access to ALL audio articles.
Vaccine development is a science over 200 years in the making, but creating a preventative that is effective and safe remains a complex and challenging task. Among the many companies working to create a vaccine for COVID-19 is Tonix Pharmaceuticals. Their pre-clinical candidate vaccine uses a unique platform that sets it apart from other efforts to fight the pandemic. We spoke to Tonix CEO Seth Lederman to find out more.
Ruairi Mackenzie (RM): Could you outline why your approach to vaccine development is different?
Seth Lederman (SL): Our vaccine program is based on a platform technology, where we're using a live replicating horsepox virus to be the vector for antigens from the SARS-CoV-2 virus. What is different about what we're doing relative to what others are doing is that our live replicating virus leads to a strong immune response that has a strong component of cellular immunity or T cell immunity, whereas the other programs are designed for the most part to induce the formation of humoral immunity or antibodies.
RM: What's the significance of having a cellular immunity produced as opposed to a humoral antibody immunity?
SL: First of all, it's not that we're producing a cellular immune response in the absence of an antibody response, but we're producing both. Whereas the other programs in development, for the most part, are really heavily focused on producing the antibody response. We don't know much about COVID-19, but from the work that was done with SARS, it's clear that people who recovered from SARS had strong T cell immunity and T cell immunity lasts years, whereas antibody immunity is relatively short-lived and relatively weak. The other problem with a pure antibody immune response is that there's a potential for something called antibody dependent enhancement (ADE). And ADE is a process in which the antibodies a person or an animal produces in response to a virus might actually be used by the virus to enter different cells than they normally would. For examples, liver cells or macrophages or other cells that have receptors for the FC portion of antibody.
RM: Is ADE common or is it a rare event?
SL: It's definitely a risk since we don't understand a lot about COVID-19 and SARS-CoV-2, but it clearly can happen. And in the case of SARS, where there was more time to study it, ADE happened in animal models. So, given the similarity of SARS to SARS-CoV-2, ADE is a real concern.
RM: How did you develop your vector and how does it work as a carrier in the vaccine?
SL: We were developing it as a vaccine to protect against smallpox and monkeypox. Smallpox was eradicated by the WHO’s accelerated vaccination program, but there is still considerable concern that rogue nations, particularly North Korea, may have smallpox and might use the malicious reintroduction of smallpox as a bioweapon. There's a lot of concern about that. I think that the fact that SARS-CoV-2 has shut down the entire western economy, given that COVID-19 has maybe a 1% mortality rate is a stark reminder of the seriousness of smallpox. Seriousness, meaning that smallpox has a 30% mortality rate. And smallpox has that kind of mortality rate in the young, healthy, middle aged, old – it’s indiscriminate. So the potential of smallpox as a bioweapon should be emphasized by the experience that we're having now.
As a standalone, our vector is a potential vaccine for smallpox. But in the case of COVID-19, it's kind of like a pickup truck and we can put into it in a modular way, a new antigen or group of antigens that would potentially protect against other infectious agents.
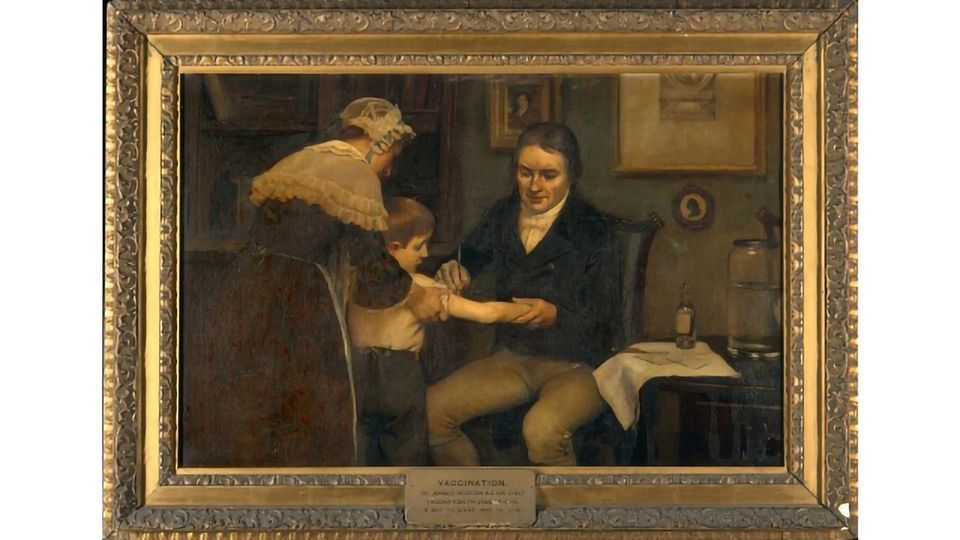
Let me take you back to 1796. Edward Jenner, in the countryside of England, found the first vaccine ever for smallpox and he called it cowpox or vaccinia. And Jenner's vaccine has been used for now 224 years and that's the vaccine that eradicated smallpox. We and others have collected compelling molecular evidence indicating that what Jenner was working with was closer to horsepox – what we're working with – than it is to modern day vaccinia. Jenner's vaccine has been propagated for 224 years separately in different countries around the world. And in these countries it evolved, particularly since it was mass produced by infecting cows. The different vaccinias have curiously evolved in the same direction even though they were evolving separately. They've had deletions and other changes.
Now we don't think that horses are the natural host for smallpox. It's more likely that the natural host is some kind of rodent, like a field mouse or a vole. There hasn't been a case of horsepox observed for more than 40 years. Horsepox basically has either gone away entirely or gone into animal reservoirs somewhere, but it hasn't attracted anyone's attention in the veterinary medicine field for more than 40 years. Presumably that's just because of good animal husbandry. In contrast, the more modern forms of the vaccinia vaccine have gone feral and have infected cows and horses in Brazil and Colombia and water buffalo in India.
RM: So the advantage of going back to something more similar to Jenner's original approach is that it's less likely to mutate into something that might spread again?
SL: That's the hypothesis under which we're operating. When people make a vaccine today, there's a concept of a master virus bank so that you're always going back to the same strain that you tested. That wasn't the case for what Jenner did because for the first 120 years after his vaccine was introduced and found to be effective, people didn't even know what a virus was. So they were clearly operating in the dark. The concept is that by going back to this vector that hasn't had these deletions and mutations, it would have the tolerability and activity that Jenner used and presumably also a decreased propensity to spread to other animals.
RM: What are the other types of approach to vaccine development that are being used in the field?
SL: I think it's a great international effort where tens, if not more than a hundred companies are working on different approaches. The bottom line is that we don't know what a successful vaccine looks like. I have much respect for everyone in the field trying to stake their expertise and do something. Now, clearly, if there had been a successful vaccine to SARS or MERS and we understood the technology to make a protective immune response, then we would just take that off the shelf and apply it to SARS-CoV-2. That's not the case. I don’t think anyone can claim knowledge or understanding about what a vaccine should look like.
Horsepox is in the group of viruses called poxvirus and poxviruses have been studied as vectors for a number of years, in addition to their well-established uses for protecting against smallpox.
I think an important distinction between what we're doing and what other people in the pox vector field are doing is that ours is a live replicating virus. There's another non-replicating vaccine called MVA, it stands for modified vaccinia Ankara. MVA was originally a poxvirus but was made by packaging it into chicken cells. It was adapted to grow in birds and lost the ability to replicate in humans. So there are at least two groups working on an MVA-based vaccine. That would be very close to what we're doing as they're also using a poxvirus and it’s also a live virus, but it's not replicating in human cells. So I think that they would say that their advantage over what we are doing is that they claim to have a tolerability advantage. I think that everyone would acknowledge ours as a replicating virus will have a stronger T cell immune component in response to it.
I think SARS was a wake-up call that the United States ignored because it mostly stayed in Asia.
RM: Have you noticed a change in attitude among different companies to sharing knowledge and resources or are companies still keeping themselves to themselves?
SL: I think the global sharing cooperation is very much in effect. The WHO is leading an effort of vaccine makers to share information on animal models and things like that. They've already been setting up video calls, so that's a great effort. The Biotechnology Innovation Organization (BIO) also has a COVID-19 effort. It’s mostly based in the United States, but there are many international companies that are part of that. I think there is cooperation. There's not yet an established animal model. That will most likely have to be a primate model because of the receptor for SARS-CoV-2. The ACE-2 receptors are very different in mice.
RM: How do you think the current outbreak will change government attitudes to vaccination?
SL: I hope that the enormity of the situation keeps government focused on the vaccine issue. I think SARS was a wake-up call that the United States ignored because it mostly stayed in Asia. But in Asia, the experience led them to have better and faster responses to COVID-19 than maybe we did. Even in the years intervening since 2003, people in Japan and people in China have often worn facemasks; it's a common sight in Asia and not common in the United States.
One of the problems we have in the United States is that without information people oppose vaccination and it's led to outbreaks of things like polio and measles that are almost entirely preventable. Given the enormity of the situation, I hope that policymakers keep their focus for a longer period of time than just after this crisis passes. I think it would be unwise to think that once this has passed, the problem will be gone.
RM: Does it seem likely that SARS-CoV-2 will become part of the repertoire of viruses that we have to deal with and provide vaccinations for each year?
SL: But I think even in the papers today, it seems that the CDC is coming around to the view that, probably, the vast majority of Americans are going to be exposed to this at some point. Which will mean that the people who aren't exposed to it short-term are likely to be exposed to it later. So I think this is more likely than not to be around for a while now. So to some extent, a protective vaccine against the coronavirus will require new technology that is yet to be understood.
Seth Lederman was speaking to Ruairi J Mackenzie, Science Writer for Technology Networks


