
POSTER
Assessing the Quality and Quantity of Cell-Free DNA
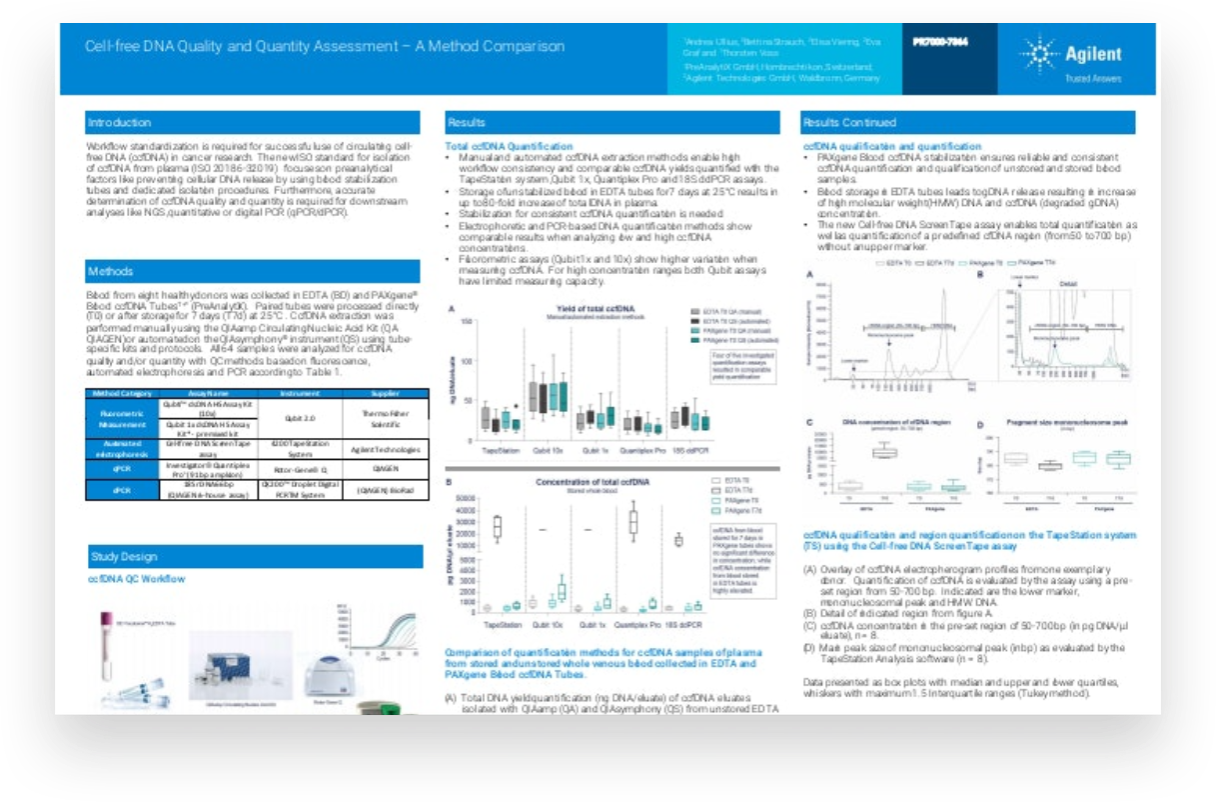

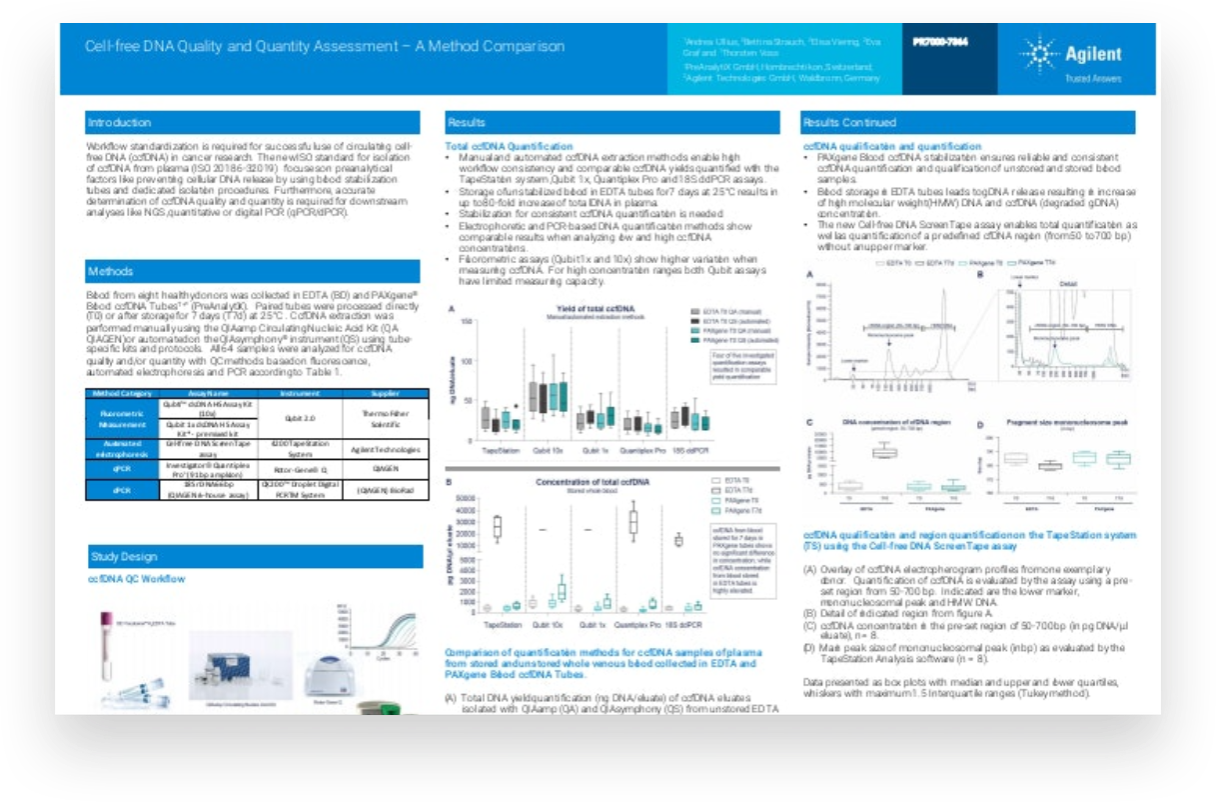
For circulating cell free DNA (ccfDNA) to be used in cancer research successfully, workflow standardization is essential. The new ISO standard for isolation of ccfDNA from plasma focuses on preanalytical factors such as preventing cellular DNA release, by using blood stabilization tubes and dedicated isolation procedures. Accurate determination of ccfDNA is necessary to ensure good quality and quantity for downstream analysis using next-generation sequencing or polymerase chain reaction techniques.
Download this poster:
- For a comparison of ccfDNA quantification and qualification methods
- To learn how to yield smaller ccfDNA fragments
- For tips on how to achieve optimal workflow control
