Screening Living Cells at High Speed

Complete the form below to unlock access to ALL audio articles.
To successfully drive cell line development, antibody discovery and gene editing fields forward, it is crucial that researchers have a full understanding of live cells and their dynamics. This knowledge ensures they are able to successfully and precisely manipulate, culture, assay and recover live cells.
We recently spoke with Eric Hobbs, CEO at Berkeley Lights to learn more about "digital cell biology". Eric also describes how optofluidics can be used to gain fast, comprehensive measurements and insights across thousands of cells in parallel.
Laura Lansdowne (LL): For some of our readers less familiar with Berkeley Lights could you tell us a little about the company mission and history?
Eric Hobbs (EH): Berkeley Lights was founded in 2011 to accelerate the discovery, development, and use of cell-based products. Today, cells are used to create therapies, super producing crops, and other biology-based products. One of the biggest challenges in the discovery, development, and manufacturing of these products is identifying the cells with the desired function. We have developed workflows that speed the assessment of the function and quality of thousands of individual cells in parallel. This lets researchers find the best cells in less time and for less money for a variety of applications. We believe that this will enable the rapid deployment of biology to solve the largest problems we face today – access to sustainable and scalable sources of food, therapies, and energy.
LL: What is “digital cell biology” and how is your technology influencing this field?
EH: “Digital cell biology” is dramatically changing how we study and engineer cells. Through the convergence of multiple disciplines – biology, technology, and information science – digital cell biology enables the collection of millions of data points in a single experiment and at an individual cell level. Machine learning enables translation of these massive amounts of data into rich phenotypic and functional insights that can speed scientific discovery and product development.
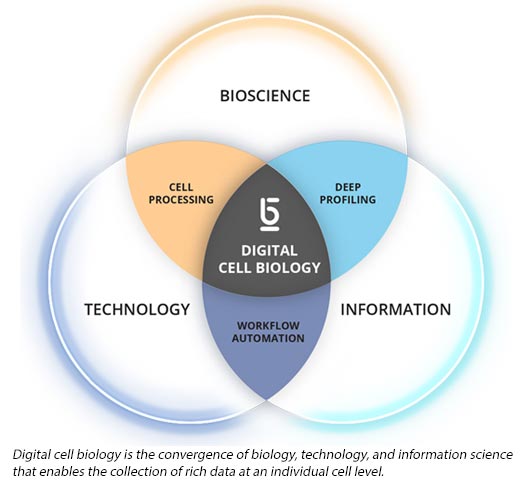
Applying digital cell biology, our platform provides more information about cell function than any other single method and enables users to run design-build-test-learn cycles on their cells significantly faster and at scales previously unthinkable. We have already demonstrated 3 to 4-month time savings in the antibody discovery and 2 to 3-month time savings in the cell line development phase of therapeutic antibody development.
Our potential to accelerate innovation and delivery of therapies has been recognized by pharmaceutical companies such as Amgen, Shire, and Novo Nordisk who are already working with our Beacon® optofluidic platform. We are now extending our applications into synthetic biology where we can help accelerate access to more cost-effective, sustainable sources of food and energy.
LL: Could you tell us more about how your optofluidic platform works?
EH: Our Beacon optofluidic platform enables massively parallel single-cell isolation, culture, assaying, and export of viable cells or clones of interest.
Cells are loaded onto our proprietary nano-volume OptoSelectTM chips, which more precisely mimic what happens when cells are used in bioreactors for production than traditional assay formats. Loading cells
Loading cells
These chips contain thousands of tiny reaction vessels called NanoPenTM chambers. Individual cells of interest or groups of cells are moved into NanoPens based on pre-defined characteristics using low intensity light cages and automated protocols.
 Culturing and exporting cells
Culturing and exporting cellsOnce cells are assembled in NanoPens, scientists can culture cells and run a variety of assays. This is achieved by flowing culture media and assay reagents in and out of the OptoSelect chip and scoring results using a brightfield and four fluorescent imaging channels. This allows measurement of a wide range of parameters including:
- cell count
- cell diameter
- cell circularity
- cell-cell interactions
- growth rate
- surface marker expression,
- IgG secretion
- multispecies binding
- RNA expression
Thus, enabling direct linking of phenotype and function. All of these data combined provide detailed cell “fingerprints” that enable scientists to select only those cells for export that have desired characteristics.
LL: What applications can the technology be used for and what are some of the benefits of using it?
EH: Anyone who is creating bio-based products can benefit from our technology. This includes pharmaceutical companies that create cell-based therapeutics and biotech companies that engineer cells to manufacture industrial chemicals, energy or food.
Currently, application workflows for the Beacon platform include cell line development and antibody discovery. As mentioned, this platform is able to provide substantial time savings for antibody discovery workflows and cell line development processes for biologics. We just launched a desktop platform to support T cell research and in the not-so-distant future we will extend our applications into synthetic biology where this technology could help accelerate access to cost-effective, sustainable sources of food and energy.
Eric Hobbs, Ph.D., CEO, Berkeley Lights, was speaking to Laura Elizabeth Lansdowne, Science Writer for Technology Networks.


