Key molecule for flu infection identified
Research Press Release | May 30, 2018
After decades of research, a research team has discovered the key receptor molecule that enhances the infection of the influenza A virus, providing a novel target for anti-flu drug development.
Viral infection starts when a virus particle attaches to a receptor molecule on the surface of a host cell. The virus particle then hijacks cellular machinery to enter the cell and replicate itself, establishing the infection. The key receptor molecule for the influenza A virus (IAV) has remained unidentified despite decades of research.
A research team led by Professor Yusuke Ohba of Hokkaido University previously demonstrated that changes in Ca2+ concentration in host cells play an important role in IAV infections.
In the latest study published in Cell Host & Microbe, the team has discovered that the Ca2+ channel, a transmembrane protein that allows Ca2+ to move across the cell membrane, is the key receptor molecule for IAV infections. Furthermore, treating human cells with calcium channel blockers (CCBs), which are commonly used as anti-hypertension drug, significantly suppressed IAV infections.
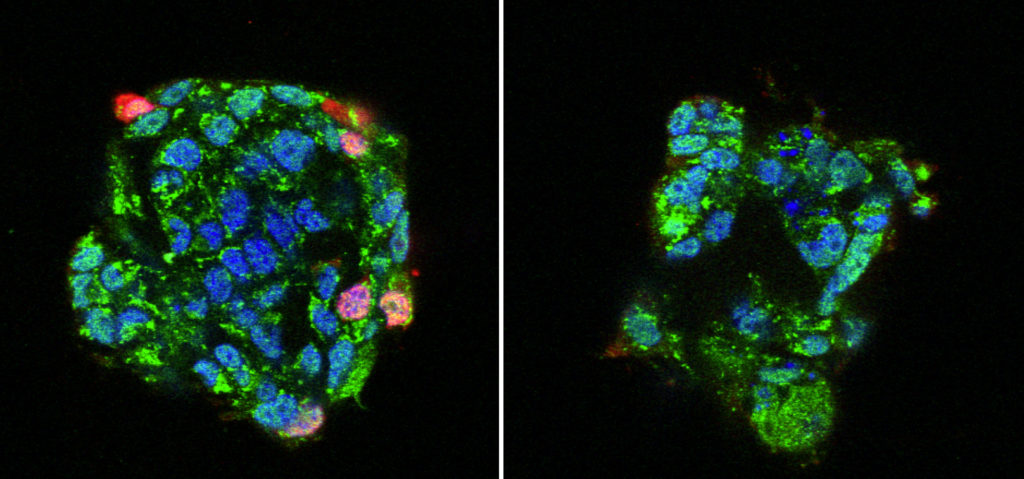
Human bronchial epithelial cells cultured with (right) or without (left) a calcium channel blocker (CCB) prior to exposure to IAV. Red signals show infected and replicated IAV. Treatment with CCB significantly suppressed IAV infections. (Fujioka Y. et al., Cell Host & Microbe, May 17, 2018)
In experiments using cultured human cells, the team found that IAV binds to the Ca2+ channel on the cell’s surface to trigger an influx of Ca2+, followed by entry of the virus and infection. Knocking down Ca2+ channels inhibited IAV-induced Ca2+ influx and virus entry. They also revealed that sialic acid on the Ca2+ channel is crucial for the virus to bind.
Cultured monkey cell showing IAV-induced Ca2+ influx (oscillations). (Fujioka Y. et al., Cell Host & Microbe, May 17, 2018)
Finally, the team tested the effect of CCB on IAV infections using mice. When they treated the animals with CCB intranasally, a significant and dose-dependent reduction in the amount of replicated viruses was observed. When the animals were treated with high amounts of IAV, administration of CCB significantly prolonged survival and allowed weight recovery of the survivors whereas the untreated group died within five days.
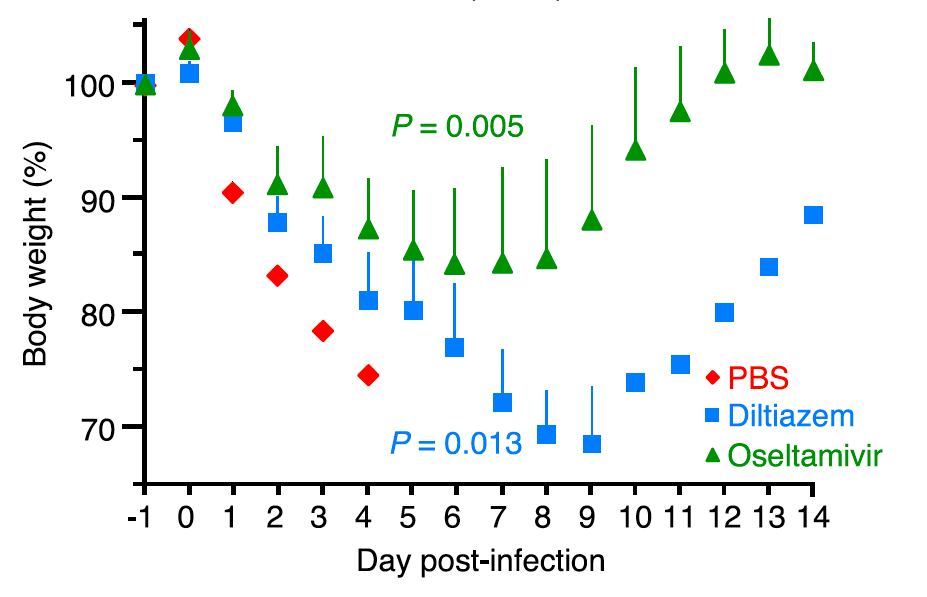
Changes in body weight after IAV infection. Mice untreated with CCB died within five days after IAV infection while the group treated with CCB (diltiazem) survived and recovered their body weights, as did a common anti-flu drug oseltamivir-treated group. (Fujioka Y. et al., Cell Host & Microbe, May 17, 2018)
“There were cases when the suppressive effect of CCB on IAV infections was comparable to that of an existing anti-flu drug. We expect that the interaction between IAV and the Ca2+ channel could be a novel and important target for future drug development,” says Yusuke Ohba.
Original article:
Fujioka Y. et al., A Sialylated Voltage-Dependent Ca2+ Channel Binds Hemagglutinin and Mediates Influenza A Virus Entry into Mammalian Cells. Cell Host & Microbe, May 17, 2018.
DOI: 10.1016/j.chom.2018.04.015
Funding Information:
This study was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (#26115701 and #15H01248), Japan Society for the Promotion of Science (#26293041 and #16H06227), Japan Agency for Medical Research and Development (JP17fk0108124j0601), Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Waksman Foundation of Japan, the Sumitomo Electric Group Corporate Social Responsibility Foundation, and SENSHIN Medical Research Foundation.
Contacts:
Professor Yusuke Ohba
Faculty of Medicine and Graduate School of Medicine
Hokkaido University
Email: yohba[at]med.hokudai.ac.jp
Naoki Namba (Media Officer)
Global Relations Office
Institute for International Collaboration
Hokkaido University
Tel: +81-11-706-2185
Email: pr[at]oia.hokudai.ac.jp

