MSC NutriStem®, a complete xeno-free, serum-free system for the growth and expansion of hMSCs
MSC NutriStem® XF Medium
REDEFINING STEM CELL EXCELLENCE AND ADVANCING CLINICAL APPLICATIONS
A defined, serum-free and xeno-free culture system optimized for the growth and expansion of hMSCs from a variety of sources: placenta (hMSC-PL), adipose-tissue (hMSC-AT), Wharton jelly (hMSC-WJ) and bone marrow (hMSC-BM). NutriStem® MSC culture system is developed specifically for the attachment, dissociation and freezing, as well as enabling long-term growth of hMSCs while retaining self-renewal and multi-lineage differentiation potential.
Defined, serum-free, xeno-free reagents are essential tools for all human mesenchymal stem cell (hMSC) research having potential clinical applications. The NutriStem® MSC Culture System includes defined reagents ideal for translational research use.
hMSCs cultured in serum-free, xeno-free MSC NutriStem® XF Medium show superior proliferation and self-renewal potential in comparison to serum-containing media and other serum-free media. In addition, hMSCs maintain their proper fibroblast-like cell morphology, tri-lineage differentiation potential, and demonstrate normal hMSC marker profiles and karyotypic stability over long-term culture.
Features
• Serum-Free (SF), Xeno-Free (XF), defined formulation
• Drug Master File (DMF) available
• hMSCs demonstrate superior proliferation
• Supports long-term growth and self-renewal of hMSCs from multiple sources
• Proper hMSC characteristics and multilineage differentiation potential are maintained over long-term culture
• hMSCs express high percentage of MSC surface markers and do not express hematopoeitic markers
• Complete media kit; no additional supplements are required
Cells cultured in serum-containing medium do not require an adaptation phase when transitioning to serum-free MSC NutriStem® XF Medium.
Initial Isolation:
hMSC from various sources (hMSC-PL, hMSC-AT, hMSC-WJ, hMSC-BM) can be efficiently isolated using MSC NutriStem® XF on pre-coated dishes. Addition of 2-2.5% human AB serum may be required for certain tissues. Using MSC NutriStem® XF for isolation of hMSC enhances purity of MSC populations in earlier passages and increases the number of hMSC in comparison to FBS-containing medium.

Expansion:
hMSC cultured in MSC NutriStem® XF exhibit a high proliferation rate and long term growth in comparison to competitors’ media (see figure 2).
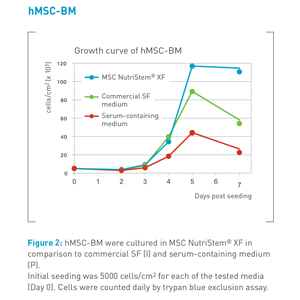
MSC NutriStem® XF is optimized for hMSC from various sources
MSC NutriStem® XF promotes proliferation of hMSC from a variety of sources while maintaining normal fibroblast-like, spindle shape cell morphology (see figure 3).

hMSC Differentiation potential
hMSC cultured in MSC NutriStem® XF maintain their trilineage differentiation potential (see figure 4).

References:
1. S. Bobis-Wozowicz et al. Diverse impact of xeno-free conditions on biological and regenerative properties of hUC-MSCs and their extracellular vesicles. Journal of Molecular Medicine, 2016
2. K.Y. Tan et al. Serum-free media formulations are cell line–specific and require optimization for microcarrier culture. Cytotherapy, 2015
3. Cai, Zhen, et al. Chondrogenesis of Human Adipose-Derived Stem Cells by In Vivo Co-graft with Auricular Chondrocytes from Microtia. Aesthetic plastic surgery 39.3 (2015): 431-439.
4. A. Jarmalavičiūtė et al., Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine–induced apoptosis. Cytotherapy, 2015
5. M.Pokrywczynska et al., Transdifferentiation of Bone Marrow Mesenchymal Stem Cells into the Islet-Like Cells: the Role of Extracellular Matrix Proteins. Archivum Immunologiae et Therapiae Experimentalis, May 2015
6. U Pivoraitė et. al., Exosomes from Human Dental Pulp Stem Cells Suppress Carrageenan-Induced Acute Inflammation in Mice. Inflammation, April 2015
7. S.H. Mei, et al. Isolation and large-scale expansion of bone marrow-derived mesenchymal stem cells with serum-free media under GMP-compliance. Cytotherapy, Volume 16, Issue 4, Supplement , Page S111, April 2014
8. Li-Yi Sun, et al.Expansion of Semi-Automatic Processed Human Adipose-Derived Stem Cells in Medium Supplemented with Autologous Serum and Antioxidants. Stem Cell Research & Therapy, 2014, 4:4
9. Allen Kuan-Liang Chen, et al.Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: Achievements and future direction. Biotechnology Advances, Volume 31, Issue 7, 15 November 2013, Pages 1032–1046
For more references please refer to http://www.bioind.com/israel/products/stem-cell-research/stem-cell-culture/human-mesenchymal-stem-cells/nutristem-msc-medium/







