Why is China Becoming a Microfluidics Manufacturing Superpower?

Complete the form below to unlock access to ALL audio articles.
For the world of diagnostics, it's an exciting time.
The development of microfluidic devices has created incredible opportunities for medical research.
With promising applications predicted for liquid biopsies, infectious disease detection and personalized medicine, it’s not surprising that the market for microfluidic devices has boomed in recent years.
Recently, Yole Développement published a comprehensive overview of the Chinese microfluidics market, which is experiencing particularly rapid growth.
To gain insights into the fascinating reasons for this, we spoke with Sébastien Clerc, Technologies & Market Analyst, Microfluidics & Medical Technologies at Yole Développement (Yole).
What is the objective of “Made in China 2025”, and what sectors will be affected? What has driven the Chinese government to invest so heavily in pharmaceuticals and medical devices?
“Made in China 2025” is a strategic plan announced in 2015 by the Chinese government which aims to increase the competitiveness of the country’s industry, especially in cutting-edge areas. The goal is to reduce China’s reliance on foreign technologies and suppliers, to develop a complete domestic supply chain and to become a manufacturing superpower.
The plan targets 10 high-tech industries, including “biopharma and advanced medical products”. In order to achieve these goals, national and regional governments direct large amounts of money into innovative Chinese companies to accelerate their development. With China having the world’s largest population, it is not surprising that China included medical devices, a high-tech sector, in the ‘Made in China 2025’ plan.
What tactics are Chinese microfluidics companies employing to help them compete with current international giants of the microfluidics world?
From the perspective of microfluidic device manufacturers, the primary objective is to learn and gain expertise in order to enhance their capability and increase the quality of their products: indeed, these companies are much younger than their Western counterparts and lack experience and know-how. In order to catch up, they offer manufacturing services at much lower cost than western companies thereby attracting customers while maintaining steady improvements.
From the perspective of the diagnostics companies, the situation is similar. The market for complex microfluidic-based diagnostic tests is dominated by foreign players, but Chinese players are proposing interesting and innovative solutions in terms of performance, and more significantly in terms of price, which should enable them to increase their market share.
Moreover, a significant proportion of these companies think that local Chinese microfluidic device manufacturers are not good enough and thus choose to produce in-house. This is made possible by bringing back experienced Chinese engineers that have worked overseas.
What rate of growth is predicted for the Chinese microfluidics industry? How does the timeline from company inception to product commercialization in China compare with that of companies elsewhere?
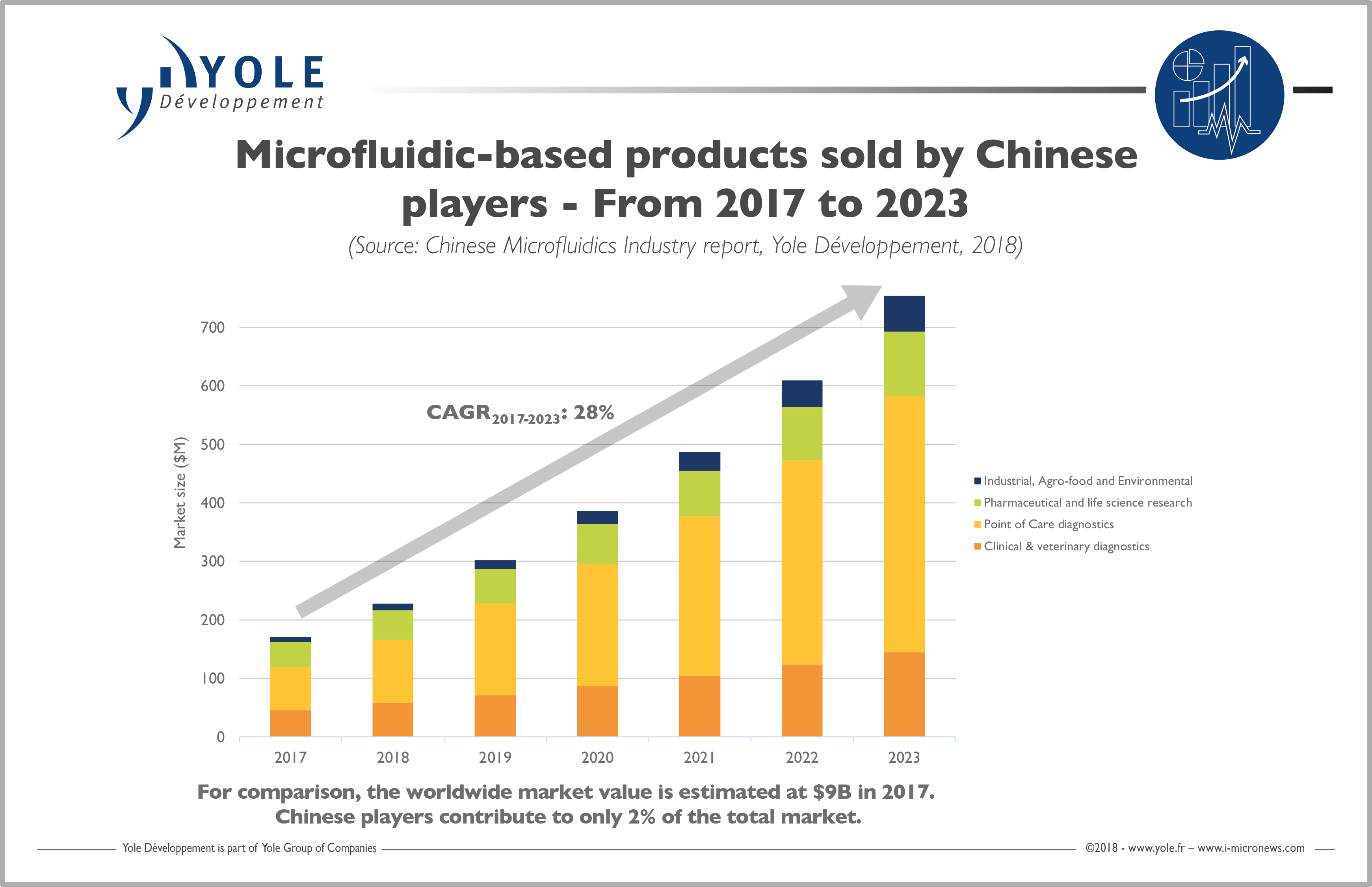
At Yole Développement (Yole), we estimate that the market for microfluidic-based products sold by Chinese players will grow at a CAGR2017-2023 of 28%, from US$171 million in 2017 to US$754.1 million in 2023. This is a much faster growth than the global microfluidics market, which is steady at around 18% annual growth. The fact is that the time to market seems much shorter for the emerging Chinese companies.
We see companies that were created in 2014 and 2015 already bringing their first products to the market. This is incredible when compared to the West where most successful products remain in the pipeline for up to 10 years before entering the market. The question is, will these new and rapidly-born products be successful? In the Western world, microfluidic companies often fail due to lack of funding and not because of bad technology. With the huge amount of funding from the government, will Chinese companies do better? We will see in the coming years.
How does the quality of microfluidic-based products in China compare to what is offered by other international companies?
The quality of Chinese-made microfluidic chips is criticized by both Chinese and Western integrators. Chinese microfluidic device manufacturers mainly produce low-end chips, and are still unable to produce complex ones, such as those that need multi-mask layers or those that need extensive back-end processing. Most Chinese microfluidic device manufacturers don’t have the capability to deliver an entire product and therefore need to enter into several partnerships to do so.
Moreover, there are still gaps in the supply chain, especially for glass and silicon microfluidic chips. Some Chinese diagnostics companies choose, therefore, to internalize the production of the microfluidic chips they use in their products, while others still prefer working with foreign microfluidic device manufacturers.
In this way, they are able to integrate chips of better quality while still offering products at lower prices than international giants. It is difficult to say how these Chinese microfluidic-based diagnostic tests will compare to Western ones in the future, but we already hear that some emerging products are meeting the end-users’ requirements, at much lower prices. More importantly, these new products stay below reimbursement thresholds in China, which is not the case for most international products.
What challenges (and opportunities) does this present to foreign players – what will they have to do to survive?
China has the world’s largest population and represents a major challenge for international diagnostics companies. The market is large and growing fast, and international companies are leading this market especially when talking about complex microfluidic tests. However, Chinese players are catching up rapidly in terms of know-how and product quality. They may soon flood the market, first with low-end products, then with more complex ones, both in China and in other countries.
If these products are good enough, they will gain market share as they are cheaper. This is a real threat for international companies which may have to cut their margins dramatically to remain competitive, not only in China but also in all other countries. However, we are not sure that the performance of Chinese products will meet these expectations. It is possible that foreign companies will stay one step ahead. In addition, foreign microfluidic device manufacturers have the opportunity to attract and tie in some customers taking advantage of the current weakness of Chinese microfluidic device manufacturers as described above. We cannot predict who will win the fight yet, but this will surely be interesting to watch!
Sébastien Clerc was speaking to Michele Wilson, Science Writer for Technology Networks.
Sébastien Clerc works as a Technologies & Market Analyst, Microfluidics & Medical Technologies at Yole Développement (Yole). As part of the Life Sciences & Healthcare division, Sébastien has authored a collection of market and technology reports dedicated to topics such as microfluidics, point-of-care, MEMS for healthcare applications and connected medical devices.
In parallel, he is involved daily in custom projects such as strategic marketing, technology scouting and technology evaluation to help academic and industrial players in their innovation processes. Thanks to his technology & market expertise, Sébastien has spoken in more than 10 industry conferences worldwide over the last 2 years.
Sébastien Clerc graduated from Grenoble Institute of Technology (Grenoble INP - Grenoble, France) with a Master’s degree in Biomedical Technologies. He then completed his courses with a Master’s degree in Innovation and Technology Management in the same institute.


