Researchers Demonstrate How Deep Learning Can Advance Study of Neural Degeneration
For Immediate Release
Researchers from North Carolina State University have demonstrated the utility of artificial intelligence (AI) in identifying and categorizing neural degeneration in the model organism C. elegans. The tool uses deep learning, a form of AI, and should facilitate and expedite research into neural degeneration.
“Researchers want to study the mechanisms that drive neural degeneration, with the long-term goal of finding ways to slow or prevent the degeneration associated with age or disease,” says Adriana San Miguel, corresponding author of a paper on the work and an assistant professor of chemical and biomolecular engineering at NC State. “Our work here shows that deep learning can accurately identify physical symptoms of neural degeneration; can do it more quickly than humans; and can distinguish between neural degeneration caused by different factors.
“Having tools that allow us to identify these patterns of neural degeneration will help us determine the role that different genes play in these processes,” San Miguel says. “It will also help us evaluate the effect of various pharmaceutical interventions on neural degeneration in the model organism. This is one way we can identify promising candidates for therapeutic drugs to address neurological disorders.”
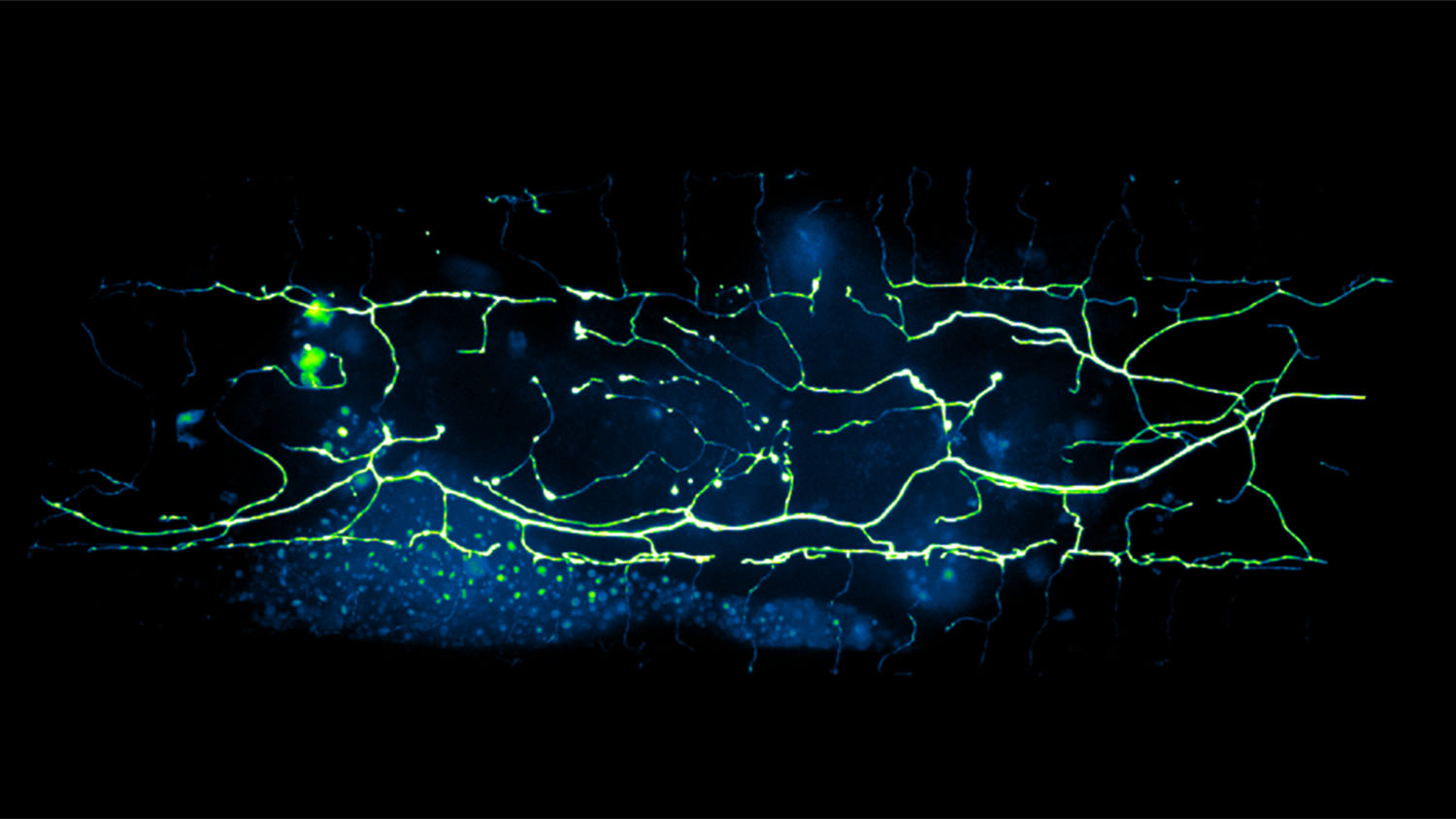
For this study, researchers focused on C. elegans, or roundworm, which is a model organism widely used to study aging and the development of the nervous system. Specifically, the researchers focused on PVD neurons, which are nerve cells that can detect both touch and temperature. The researchers chose the PVD neuron because it is found throughout the nervous system of C. elegans and it is known to degenerate due to aging.
Roundworms are tiny and transparent – meaning that it is possible to see their nervous systems while they are still alive. Traditionally, identifying degeneration in C. elegans neurons requires researchers to look for microscopic changes in the cell, such as the appearance of bubbles that form on parts of individual neurons. Researchers can analyze the extent of neural degeneration by tracking the size, number and location of these bubbles.
“Counting these bubbles is a time-consuming and labor-intensive process,” says Kevin Flores, co-author of the study and an assistant professor of mathematics at NC State. “We’ve demonstrated that we can collect all of the relevant data from an image in a matter of seconds, by combining the power of deep-learning with the advanced speed of so-called GPU computing. This enables a much faster quantitative assessment of neuronal degeneration than traditional techniques.”
In addition to monitoring the effects of age on neural degeneration, the researchers also examined the effects of “cold shock,” or prolonged exposure to low temperatures. The researchers were surprised to learn that cold shock could also induce neural degeneration.
“We also found that neural degeneration caused by cold shock had a different pattern of bubbles than the degeneration caused by aging,” San Miguel says. “It is difficult or impossible to distinguish the difference with the naked eye, but the deep learning program found it consistently.
“This work tells us that deep learning tools are able to spot patterns we may be missing – and we may be just scratching the surface of their utility in advancing our understanding of neural degeneration.”
The paper, “Deep learning-enabled analysis reveals distinct neuronal phenotypes induced by aging and cold-shock,” is published in the journal BMC Biology. First author of the paper is Sahand Saberi-Bosari, a recent Ph.D. graduate of NC State.
The work was done with support from the National Institutes of Health, under grants R00AG046911 and R21AG059099; and from the National Science Foundation, under grant IOS1838314.
-shipman-
Note to Editors: The study abstract follows.
“Deep learning-enabled analysis reveals distinct neuronal phenotypes induced by aging and cold-shock”
Authors: Sahand Saberi-Bosari, Kevin B. Flores and Adriana San-Miguel, North Carolina State University
Published: Sept. 23, BMC Biology
DOI: 10.1186/s12915-020-00861-w
Abstract:
Background: Access to quantitative information is crucial to obtain a deeper understanding of biological systems. In addition to being low-throughput, traditional image-based analysis is mostly limited to error-prone qualitative or semi-quantitative assessment of phenotypes, particularly for complex subcellular morphologies. The PVD neuron in C. elegans, which is responsible for harsh touch and thermosensation, undergoes structural degeneration as nematodes age characterized by the appearance of dendritic protrusions. Analysis of these neurodegenerative patterns is labor-intensive and limited to qualitative assessment.
Results: In this work, we apply deep learning to perform quantitative image-based analysis of complex neurodegeneration patterns exhibited by the PVD neuron in C. elegans. We apply a Convolutional Neural Network algorithm (Mask R-CNN) to identify neurodegenerative sub-cellular protrusions that appear after cold-shock or as a result of aging. A multiparametric phenotypic profile captures the unique morphological changes induced by each perturbation. We identify that acute cold-shock-induced neurodegeneration is reversible and depends on rearing temperature, and importantly, that aging and cold-shock induce distinct neuronal beading patterns.
Conclusion: The results of this work indicate that implementing deep learning for challenging image segmentation of PVD neurodegeneration enables quantitatively tracking subtle morphological changes in an unbiased manner. This analysis revealed that distinct patterns of morphological alteration are induced by aging and cold-shock, suggesting different mechanisms at play. This approach can be used to identify the molecular components involved in orchestrating neurodegeneration and to characterize the effect of other stressors on PVD degeneration.


