Mass Spec Technology Drives Innovation Across the Biopharma Workflow
Complete the form below to unlock access to ALL audio articles.
Vicki Glaser
With greater resolving power, analytical speed, and accuracy, new mass spectrometry technology and techniques are infiltrating the biopharmaceuticals workflow, from applications in biomarker identification for new drug target discovery, to validation of molecular activity, and downstream in manufacturing and quality control. The rapidly growing biosimilars industry is also leveraging the analytical capabilities and increasingly automated and easy to use MS systems designed for biopharma applications.
Thermo Fisher Scientific specifically targeted the biopharma sector with the introduction of its Q Exactive™ BioPharma MS/MS Hybrid Quadrupole-Orbitrap mass spectrometry (MS) system at the recent ASMS annual meeting. The high resolution of the Orbitrap mass analyzer makes it possible to perform MS analysis of intact biomolecules such as MAbs and to profile antibody glycoforms in their native state, Dr. Jonathan Josephs, Ph.D., director of Marketing, Pharma/BioPharma, Life Sciences MS at Thermo Scientific, tells us. The company added a third scan mode to the Q Exactive in developing the BioPharma system: a high mass range mode that provides isotopic resolution and enables intact protein analysis under native and denaturing conditions. The two existing scan modes can perform subunit and top/middle-down analysis or peptide mapping. To characterize therapeutic MAbs using intact protein analysis under native MS conditions, the Q Exactive MS can be linked to size exclusion chromatography using Thermo Scientific's Vanquish™ UHPLC system and MAbPac™ SEC-1 column.
Validating Sequence, Structure, Sugars, and Bonds
For analyzing biopharmaceuticals -- proteins, peptides, monoclonal antibodies, and nucleic acid-based therapeutics -- the industry workhorse from a mass spec-based perspective has been electrospray ionization (ESI) MS. ESI quadrupole-time-of-flight (QTOF) MS is a well-established technology for various biopharma applications, notes Bob Galvin, PhD, Vice President of the Biopharma Business Unit at Bruker Daltonics. However, whereas ESI instruments are better for some tasks, he states that "MALDI systems are becoming increasingly relevant and can solve some complementary analytical bipharma problems that ESI cannot." As examples, Dr. Galvin describes MALDI workflows for full sequence confirmation and post-translational modification (PTM) analysis of a monoclonal antibody (MAb) subunits formed by IdeS digestion, which are approximately 23-27 kDa or the determination of disulfide bond scrambling that often occurs in MAbs.
A bottom-up approach is often employed to analyze the structure of a complete 150-kDa MAb, using trypsin digestion to break the molecule into smaller peptides, typically up to 6 kDa. Tryptic digestion can induce artifact formation such as deamidation or scrambling, making it difficult to deduce an accurate picture of the original MAb from the peptides.
To overcome these challenges, he says, scientists are moving to a workflow that combines ESI-QTOF MS with the use of enzymes that produce fewer fragments from a digested MAb fragment, such as the FabRICATOR® enzyme from Genovis AB. It cleaves an IgG molecule at the hinge region, where the heavy and light chains meet, producing three fragments: the Fc/2, Fd, and LC fragments. The enzyme does not introduce any artifacts.
Bruker's maXis II ETD QTOF system with Electron-Transfer Dissociation (ETD) capabilities has the resolving power and accurate mass at online liquid chromatography speeds to analyze a 27-kDa subunit formed and to find the monoisotopic mass, according to Dr. Galvin. "Determining the monoisotopic mass of all of the three MAb fragments goes a long way to suggesting you have the right structure," he states. Additionally, whereas top-down proteomic approaches that combine ETD and CID fragmentation information can obtain about 80% sequence coverage of each fragment, using complementary MALDI-TOF/TOF MS "we can nearly always validate 100% of the sequence of each fragment," says Dr. Galvin.
Furthermore, a system that can determine monoisotopic molecular weight can also detect small, but important changes down to below 1 mass unit within the 27 kDa subunits. Biopharma companies can use this capability to monitor changes occurring within the MAb, such as quantifying the degree of deamidation. "Deamidation is a very important critical quality attribute because it affects efficacy and stability, and hence the shelf-life of a product," adds Dr. Galvin.
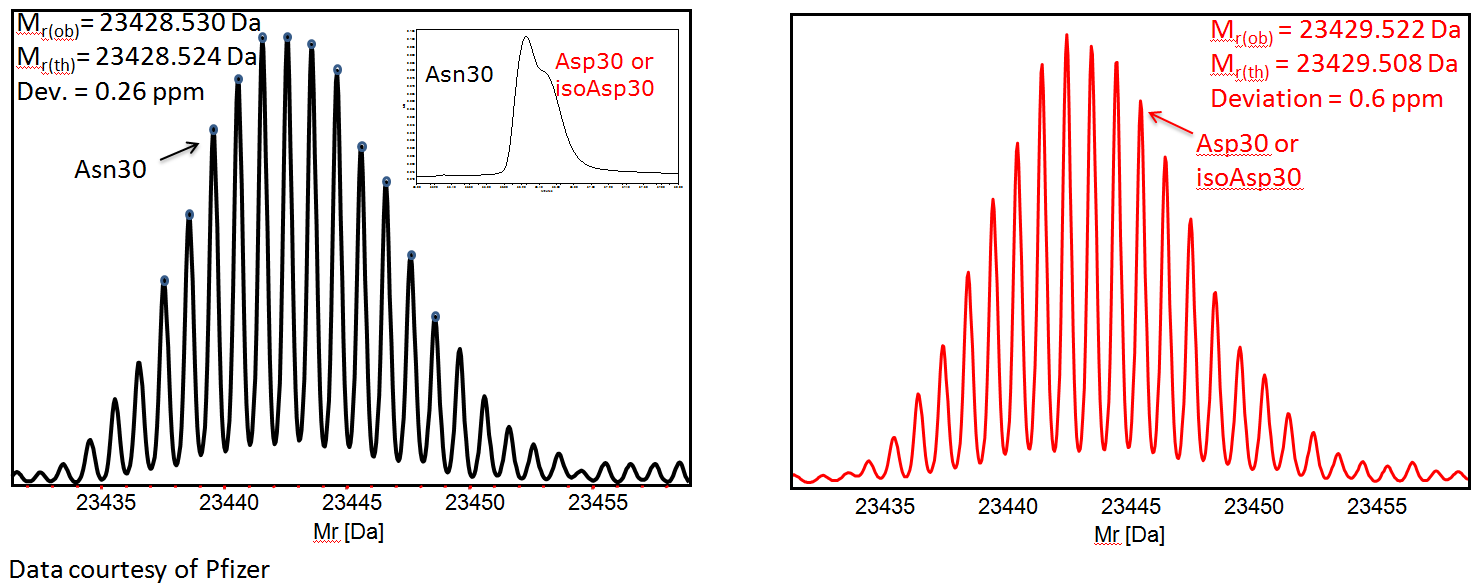
Deamidation screening – spectral interpretation based on averaging scans acquired at the front and tail of Light Chain doublet from HPLC separation. Dmass = +0.992 Da
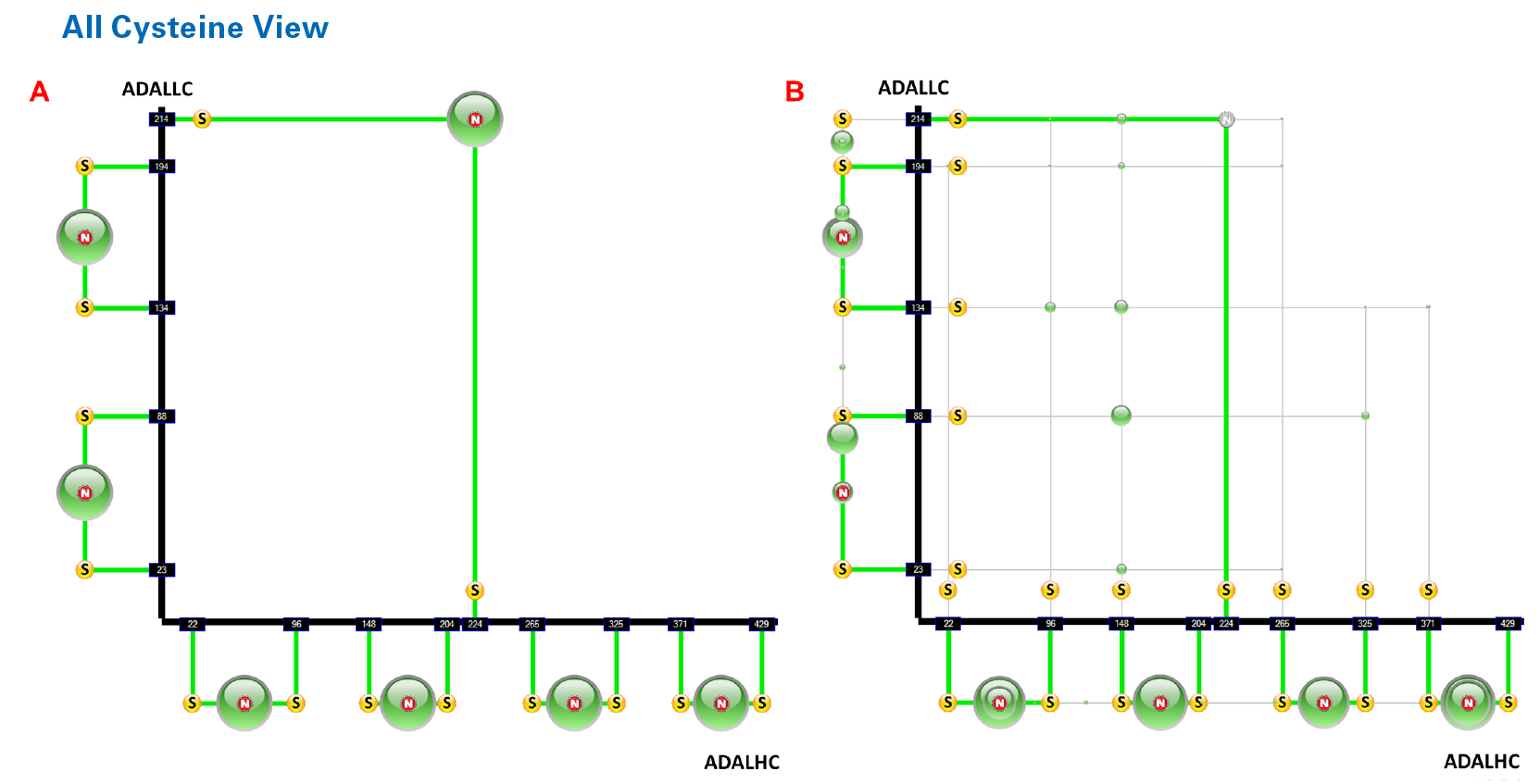
A) Graphical representation of the native bonds of adulimumab. The size of the circle represents the relative intensity compared to all DSBs. An “N” in the circle shows it is a “native” bond.
Biosimilars Demand Intensive Characterization
The race to develop biosimilar drugs has captured the attention of biopharma companies in countries worldwide and is catalyzing growing interest in MS technology, not only in the Western world but increasingly in developing regions as well, John Gebler, Ph.D. Director of Biopharma Business Development at Waters, tells us. From a workflow standpoint, even though companies such as Waters can offer an essentially end-to-end LC-MS workflow, the technology is still rather sophisticated and requires a certain skill set to use effectively.
"There is a desire [among our customers] to simplify the ability to run this technology," he says. Overall, customers are telling Waters that they want to use MS as a tool to get the answers they need. They are mainly interested in the results and not necessarily in more features and options or the ability to intervene in the automated workflows and perform higher level functions. One of the biggest bottlenecks they are facing is how to interpret and make use of the huge amounts of data modern MS samples can generate. Together with a desire for more automation to reduce manual intervention and human error and to ensure a well-defined workflow, "there is an expectation that the informatics will be there to interpret the data at the back-end," says Dr. Gebler.
The development of a biosimilar requires extensive analytical characterization of both the innovator product and the biosimilar. MS techniques can be used to obtain direct measurements of complex biopharmaceuticals such as peptides. The MS data support sequence confirmation, structural analysis (e.g., number and location of disulfide bonds), and identification and quantification of post-translational modifications (e.g., glycosylation patterns). Dr. Gebler describes this area as "a moving target," in part due to the continuing evolution of the technologies and changing regulatory requirements as authorities gain more experience with biosimilar drug submissions. Furthermore, developers of innovator drugs modify and improve their manufacturing processes throughout the lifetime of a drug, at least in part to make it more difficult for the biosimilars industry.
"The menu of what people consider to be critical attributes is growing," says Dr. Gebler. More intensive characterization of drug compounds and the identification of more, and more diverse, critical attributes that must be met in manufacturing, can improve the reproducibility of the manufacturing process. It can also help companies strengthen the intellectual property surrounding an innovator drug and offer greater protection against competition from biosimilars. The more intensive characterization of biopharmaceuticals enabled by MS technology is helping to fuel this trend.
Dr. Gebler also describes the trend toward increasing use of MS technology in the QC stage of biopharma manufacturing. "Companies are getting really good at watching and controlling their processes, but they struggle with how to look directly at their product," he says. With the trend away from batch processing to continuous manufacturing, companies want to be able to monitor their product in as close to real-time as possible -- and to look at multiple attributes in each sample.
"The use of MS in QC is a hot topic," Dr. Gebler says, " there is a very healthy discussion going on in biopharma now about what level of MS is needed -- a simple or high-resolution system -- and what information is desirable and meaningful," in terms of understanding and improving the manufacturing process. This is especially relevant for biosimilars, he explains, because there is currently no clear definition of "similar," or of how a biosimilar can differ from the innovator product.
Biomarker Validation
MS technology is playing an increasingly important role in the identification and validation of biomarkers for use in biopharmaceutical drug development. Biomarkers are being used to drive the discovery of new drug targets, to confirm the activity of targeted molecules, detect off-target effects, and monitor therapeutic response. They are also becoming a critical tool in clinical research to aid in selecting patients for clinical studies, understand drug response, and develop personalized therapies for precision medicine.
Agena Bioscience™ describes a variety of genomics-based applications of its MassARRAY® System for performing MALDI Tof MS, including for cancer profiling of solid tumors and liquid biopsies, in pharmacogenomics for testing ADME genes and assessing risk of drug-induced complications, and in clinical research. In pharmacogenomics, for example, MS offers an alternative to microarrays or next-gen sequencing for genotyping to identify single nucleotide polymorphisms (SNPs) associated with greater likelihood of sensitivity or resistance to a drug compound. Chen et al. used the Agena Bioscience MassARRAY System for genotype analysis of samples from patients with non-small cell lung cancer to detect 13 SNPs in eight genes previously associated with response to platinum-based chemotherapy (Oncotarget 2016;7[34]:55741-55756). The researchers identified specific polymorphisms in two genes that were associated with either greater sensitivity or resistance to the platinum-based chemotherapy.
In another example, MS was used to identify a biomarker for risk of radiation-induced fibrosis in patients with breast cancer. In a recent publication, Weigel et al. suggest that the biomarker could also serve as a therapeutic target for preventing fibrosis in patients at risk (Nature Comm 2016;doi:10.1038/ncomms10893). The authors performed differential genome-wide DNA methylation analysis using MassARRAY technology, comparing primary human dermal fibroblasts from breast cancer patients treated with radiation in whom fibrosis did or did not develop. The results pointed in particular to the fibrosis-associated hypomethylation of a kinase gene enhancer that contained two differentially methylated sites. Determination of methylation status at this site prior to treatment could help predict fibrosis risk and guide individualized dosing. A drug designed to block the affected kinase might prevent the profibrotic effects of radiotherapy.
Conclusion
Offering analytical capabilities that were not readily available outside of the basic research setting, MS technology slowly found its way into biopharma laboratories through skilled operators capable of using the instruments for characterizing biomolecular drug candidates. As the MS industry is designing increasingly automated, user-friendly systems with software to manage and interpret the large volumes of data produced, the spotlight focused on mass spec for analyzing complex biopharmaceuticals shines brightly. In the surging biosimilars industry, in particular, MS is an increasingly valuable analytical tool.

