Meet the Culprits of Cell Culture Contamination

Complete the form below to unlock access to ALL audio articles.
The air is warm and humid, there is an abundance of food, and your friends come and go with their shiny toys. What sounds like a dreamy summer holiday is also the reality of in vitro cell culture experiments, and a golden opportunity for contaminants to intrude. Every person, reagent, and piece of equipment in the laboratory is a potential vehicle for invasive microbes, unwelcome cells and chemical impurities, which can create costly issues in both bench research and manufacturing. Cell culture contamination is a problem on many levels, creating immediate implications for experiments and wider issues for the scientific community.
Consequences of cell culture contamination
Contaminants can affect all cell characteristics (e.g. growth, metabolism, and morphology) and contribute to unreliable or erroneous experimental results. Cell culture contamination will likely create a need for experiments to be repeated, resulting in frustrating time delays and costly reagent wastage. Data derived from undetected contaminated cultures can end up published in scientific journals, allowing others to build hypotheses from dubious results. The pervasiveness of cross-contaminated and misidentified cell lines is a decades-long issue; in 1967, cell lines thought to be derived from various tissues were shown to be HeLa cells, a human cervical adenocarcinoma cell line.1 However, studies involving these misidentified cell lines continued to feature in hundreds of citations during the early 2000s.2
This pattern is a well-acknowledged problem and threatens to undermine scientific integrity. The first published retraction in Nature Methods was due to cell line contamination3, and one conservative estimate of “contaminated” literature in 2017 found 32,755 articles reporting on research with misidentified cells.4 While many scientists may have been blissfully ignorant in the past, awareness of misidentified cell lines is growing.
Deciding how best to deal with this knowledge is not straightforward and has been discussed extensively.4 In the interest of preventing further data contamination, a certificate of authentication of the origin and identity of human cells is now required by the International Journal of Cancer, and encouraged by funding agencies. Others have questioned whether mandatory testing really is the best way forward.3
But what should be done about existing contaminated literature? Mass retraction of affected articles may disproportionately punish the careers of a few scientists, and could be a waste of resources containing potentially valuable data. One recently proposed system of “self-retraction” recommends replacing blame with praise in order to encourage self-correction.5 Post hoc labeling of published articles in the form of an “expression of concern” allows existing findings to remain accessible, while giving readers a chance to form their own judgement.
Lastly, pathogens carried by cells (either intentionally or accidentally) or in components of the culture medium are potential health hazards, and laboratory-acquired viral infections have been reported.6-8 Indeed, the stakes are higher when cells are to be introduced into patients, highlighting the critical importance of quality control in cell therapies.
Top 10 Tips to Avoid Contamination in Pipetting

While pipetting is a key part of everyday laboratory work, it is also one of the stages most prone to contamination. As sample contamination can affect the reliability of results, it is important to know how it can be avoided, saving both time and money. Download this poster for ten tips to avoiding contamination in pipetting.
Download PosterSponsored Content
Tips for avoiding cell culture contamination
The first step in avoiding cell culture contamination lies in being aware of potential sources, and building practices that reduce the risk of contamination from those sources.6
Before being allowed to work in a tissue culture facility, laboratory personnel should be given full practical training in aseptic cell culture techniques by an experienced staff member. While each laboratory will have their own standard operating procedures related to use of incubators, autoclaving, labeling of cultures, media storage, and waste disposal, guidelines typically include the following tips:9
● Wash or disinfect hands before and after handling cells
● Avoid leaving your cultures out of the incubator for extended periods
● Label all cultures clearly and unambiguously
● Disinfect work surfaces before and after use
● Check disinfectants are effective and appropriate choices for the job
● Work with only one cell culture at a time
● Use separate media and reagents for each individual cell line
● Quarantine new cell lines until tested negative for mycoplasma
● Avoid overusing and relying on antibiotics
● Record how long a cell line has been kept in culture
The design of the laboratory can also play a role; cabinets should be placed away from through-traffic, doors and air-conditioning inlets.6 Restricting area access to allow only essential laboratory personnel to enter reduces disturbances of airflow around the microbiological safety cabinet.
Water baths, CO2 incubators, shelves and water pans are common culprits and should be cleaned or autoclaved regularly, using a chemical disinfectant where appropriate. Other routes of infection include accidental spillages, contact with non-sterile surfaces, splash-back from pipetting or pouring, microscopic aerosol, and infestation by vertebrates, dust and mites.
Research groups isolating stem cells use unique cell properties to filter out undesired cells, explains Dr Mei-Ju Hsu, postdoctoral researcher in stem cell therapy at Leipzig University. Dr Hsu notes that: “one of the most important features of mesenchymal stem cells is the attachment and growth on the plastic surfaces without prior coating. This step serves as a good way to eliminate the non-adherent cells (e.g. blood cells) by the removal of supernatants.”
Organoid researcher Hans Clevers, from the Hubrecht Institute for Developmental Biology and Stem Cell Research at Utrecht University, assesses genetic diversity in cells through the use of single nucleotide polymorphism (SNP) genotyping. The Clevers laboratory recently branched out from their work with mammalian cells to produce snake venom gland organoids. Dr Clevers notes that: “We have come to realize that contamination of organoid cultures is a serious problem. We have observed that organoid cultures that are commonly used and are “fast growers” contaminate slower growing organoid cultures. Typical “fast growers” are the original mouse “mini-guts” that have popped up in various human organoid cultures in the lab. We SNP-type all human samples when they come in, which allows us to follow purity of human organoid cultures over time. Cheap, fast and crucial to avoid big mistakes.”
Types and sources of biological contamination
Bacteria, molds, and yeast are biological contaminants that can manifest from innumerable sources, and generally sit at the more “detectable” end of the spectrum. In contrast, prokaryotic organisms, known as mycoplasma, cannot be detected by the naked eye or typical light microscopy, and are small enough to pass through filters designed to sterilize cell culture media.10 While mycoplasmas used to arrive via contaminated fetal bovine serum and other media additives, such products from reputable sources are now checked and come contamination-free. Now, mycoplasmas are believed to be distributed from one culture to another.11 Mycoplasma detection is often outsourced, as the need to bring in a positive control presents a risk to the laboratory affected.10
Multiple approaches across PCR-based methods, indirect staining and an agar and broth culture have been recommended for tissue culture laboratories looking to test for the presence of mycoplasmas.10 Viruses can originate from patients, host animal cell sources, and in animal-derived media, and while next-generation sequencing can aid with detection,12 biosecurity laws aim to prevent potentially damaging products from being imported in the first place.
Mycoplasma Facts: Dangers of Contamination in Cell Culture Labs
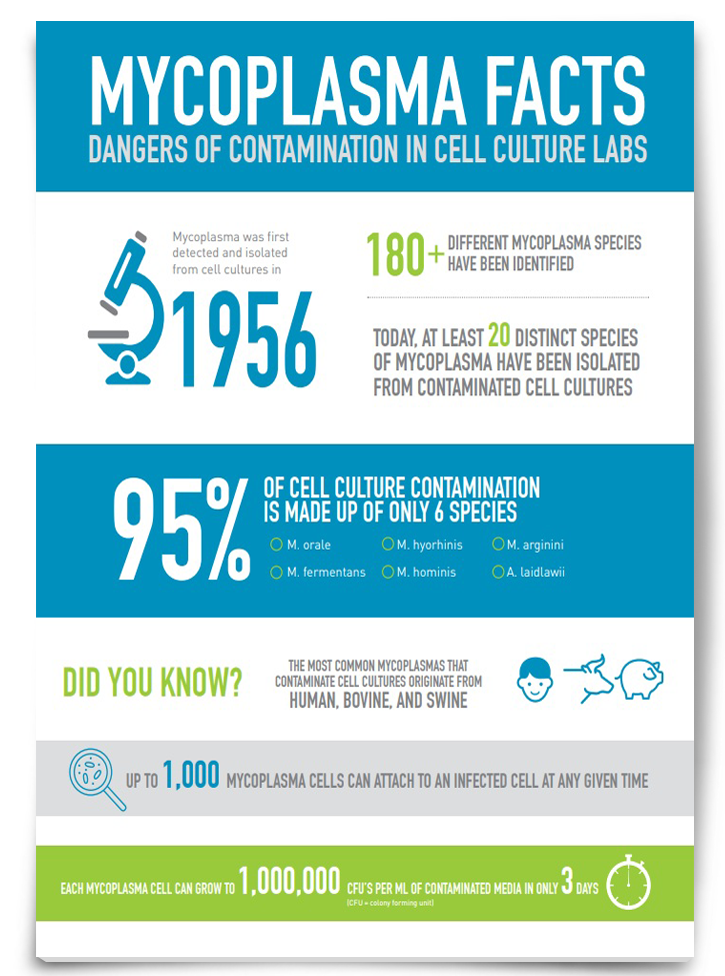
Mycoplasma is one of the most common cell culture contaminants, with six species of mycoplasma accounting for 95% of all contamination. Therefore, it is important to improve our understanding of where mycoplasma contamination can stem from and how best to prevent it. Download this infographic to discover more about mycoplasma contamination in cell culture labs.
Sponsored Content
Sources of chemical contaminants
Non-living components present in cell culture are classified as “chemical contaminants”. Chemical contaminants can be derived from reagents and equipment, or even biological sources (e.g. free radicals and endotoxins). Organic compounds, endotoxins and trace concentrations of metal ions can be present in water that has not been sufficiently purified, whereas highly purified water will draw out these components from glassware, tubing and pipes over time.13 Contaminants can be introduced during routine maintenance procedures, such as in steam generators. Ironically, chemical disinfectants and detergent washes can leave cytotoxic residues – cap liners of bottle closers are common culprits.13 Excessive exposure to fluorescent lights can lead to photoactivation of some media components, and a gradual deterioration in the quality of the media.14 Other factors not typically considered in the realm of “contaminants” include temperature, radiation, irradiation and vibration.
Rapid microbial testing, automation, and one golden question
Rapid microbial testing technologies are being developed to meet the stringent requirements for cell therapy products, which can have a short shelf-life and small sample sizes available for testing. Dr Hsu says that: “traditional methods include PCR for genes of mycoplasma and DAPI staining, but the latter is not really sensitive enough. There are now real-time PCR kits available for the detection of bacterial contamination for cell therapy products.”
Closed bioreactor systems with integrated robotics technology will be optimized to reduce contamination risk, favored by the impending trend of “scaling out” rather than “scaling up”.15,16
Regardless of the scale of your experiment, one key question to ask when troubleshooting is “what is new?” – this can help to identify possible routes of contaminant entry.
Before the culprits of cell culture contamination introduce themselves, it pays to know exactly what you are looking for; reading up on guides that show you how to identify problems with your cell culture is a good place to start.
References:
- Gartler, S. M. (1967). Genetic markers as tracers in cell culture. National Cancer Institute Monograph, 26, 167–195.
- Nardone, R. M. (2008). Curbing rampant cross-contamination and misidentification of cell lines. BioTechniques, 45(3), 221–227. https://doi.org/10.2144/000112925
- Evanko, D. (2013). A retraction resulting from cell line contamination: Nature Methods. Retrieved from http://blogs.nature.com/methagora/2013/09/retraction_resulting_from_cell_line_contamination.html
- Horbach, S. P. J. M., & Halffman, W. (2017). The ghosts of HeLa: How cell line misidentification contaminates the scientific literature. PLOS ONE, 12(10), e0186281. https://doi.org/10.1371/journal.pone.0186281
- Fanelli, D. (2016). Set up a ‘self-retraction’ system for honest errors. Nature, 531(7595), 415–415. https://doi.org/10.1038/531415a
- Geraghty, R. J., Capes-Davis, A., Davis, J. M., Downward, J., Freshney, R. I., Knezevic, I., Lovell-Badge, R., Masters, J. R. W., Meredith, J., Stacey, G. N., Thraves, P., & Vias, M. (2014). Guidelines for the use of cell lines in biomedical research. British Journal of Cancer, 111(6), 1021–1046. https://doi.org/10.1038/bjc.2014.166
- Hummeler, K., Davidson, W. L., Henle, W., LaBoccetta, A. C., & Ruch, H. G. (1959). Encephalomyelitis Due to Infection with Herpesvirus simiae (Herpes B Virus): A Report of Two Fatal, Laboratory-Acquired Cases. New England Journal of Medicine, 261(2), 64–68. https://doi.org/10.1056/NEJM195907092610203
- Coelho, A. C., & García Díez, J. (2015). Biological Risks and Laboratory-Acquired Infections: A Reality That Cannot be Ignored in Health Biotechnology. Frontiers in Bioengineering and Biotechnology, 3. https://doi.org/10.3389/fbioe.2015.00056
- Coecke, S., Balls, M., Bowe, G., Davis, J., Gstraunthaler, G., Hartung, T., Hay, R., Merten, O.-W., Price, A., Schechtman, L., Stacey, G., & Stokes, W. (2005). Guidance on Good Cell Culture Practice: A Report of the Second ECVAM Task Force on Good Cell Culture Practice. Alternatives to Laboratory Animals, 33(3), 261–287. https://doi.org/10.1177/026119290503300313
- Young, L., Sung, J., Stacey, G., & Masters, J. R. (2010). Detection of Mycoplasma in cell cultures. Nature Protocols, 5(5), 929–934. https://doi.org/10.1038/nprot.2010.43
- McGarrity, G. J. (1976). Spread and control of mycoplasmal infection of cell cultures. In Vitro, 12(9), 643–648. https://doi.org/10.1007/BF02797464
- Richards, B., Cao, S., Plavsic, M., Pomponio, R., Davies, C., Mattaliano, R., Madden, S., Klinger, K., & Palermo, A. (2014). Detection of Adventitious Agents Using Next-Generation Sequencing. PDA Journal of Pharmaceutical Science and Technology, 68(6), 651–660. https://doi.org/10.5731/pdajpst.2014.01025
- Lincoln, C. K., & Gabridge, M. G. (1998). Chapter 4 Cell Culture Contamination: Sources, Consequences, Prevention, and Elimination. In Methods in Cell Biology (Vol. 57, pp. 49–65). Elsevier. https://doi.org/10.1016/S0091-679X(08)61571-X
- Wang, R. J. (1976). Effect of room fluorescent light on the deterioration of tissue culture medium. In Vitro, 12(1), 19–22. https://doi.org/10.1007/BF02832788
- Kino-Oka, M., Ogawa, N., Umegaki, R., & Taya, M. (2005). Bioreactor Design for Successive Culture of Anchorage-Dependent Cells Operated in an Automated Manner. Tissue Engineering, 11(3–4), 535–545. https://doi.org/10.1089/ten.2005.11.535
- Wendt, D., Riboldi, S. A., Cioffi, M., & Martin, I. (2009). Potential and Bottlenecks of Bioreactors in 3D Cell Culture and Tissue Manufacturing. Advanced Materials, 21(32–33), 3352–3367. https://doi.org/10.1002/adma.200802748


