Struggling to Zero in on a Biobanking LIMS? Here is the Checklist of Essential Features To Consider

Complete the form below to unlock access to ALL audio articles.
This year, more than ever, has underscored the critical services that biobanks provide to biomedical researchers and other scientists. Biobanks are complex operations that manage thousands, or even millions, of biological samples and associated data required for scientific research.
Biobanks face many challenges
Most biobanks face the logistical nightmare of needing to collect, transport, process, store and ship samples from many different sources to scientists in various locations. All samples and data must be kept safe and secure throughout this entire process. Moreover, biobank staff must manage their own safety when dealing with biological materials and chemical hazards, such as liquid nitrogen.
Regulatory hurdles can be particularly challenging for biobanks storing samples and data, known as protected health information (PHI), from human patients. To protect the privacy of donors, biobanks must abide by a number of different regulatory guidelines, including the Health Insurance Portability and Accountability Act of 1996 (HIPAA) in the U.S. and the General Data Protection Regulation (GDPR) in the European Union (1,2). These guidelines cover many aspects of biobanking, including managing donor consent, anonymizing samples and clinical data and limiting data access to authorized users.
Another major challenge faced by biobanks is that biological samples are temperature-sensitive, and may be affected by changes in humidity, light and oxygen levels. Therefore, biobanks must build complex infrastructure and monitoring systems to maintain sample quality. By following best practice guidelines, such as ISO 20387:2018 and ISBER Best Practices (3,4), biobanks can ensure that the sample quality remains high.
How biobanks can overcome these challenges
By implementing a regulatory-compliant, configurable Laboratory Information Management System (LIMS), biobanks can follow best practice guidelines and overcome logistical, regulatory and safety challenges. A LIMS can help biobanks standardize procedures, document staff training and ensure biobank staff adhere to validated standard operating procedures (SOPs). This will ultimately maintain sample quality and integrity as well as improving workflow efficiency and staff safety.
Choosing the Right Biobanking LIMS
Before choosing a biobanking LIMS, also known as biobank management software, biobank managers should look for the following features and capabilities:
1. Manage the Entire Sample Lifecycle
For a LIMS to be effective, it must be able to manage the entire sample lifecycle, from collection and donor consent, right through to monitoring transport, processing and storage conditions, associating samples with clinical data and documenting disposal of samples. A LIMS should integrate with all automated systems used by biobanks, such as automated storage, temperature monitoring systems or liquid handling systems, and update data, including sample storage location, in real-time to facilitate interoperability.
2. Adapt to the Biobank’s Needs
Biobank staff should be able to create and configure data fields within a LIMS, including sample data, patient data, sample storage locations and test results.
If a biobank’s clients conduct multisite studies, a LIMS must be able to harmonize and integrate data from different sources. For biobanks with multiple clients, a LIMS must be able to track and manage samples stored in different conditions, for example, at -20°C, -80°C or in liquid nitrogen, and processed for different assays, such as RNA, DNA or protein assays. Furthermore, a LIMS should be capable of tracking and managing legacy/archived samples as well as active samples from multiple studies, while maintaining strict privacy and security.
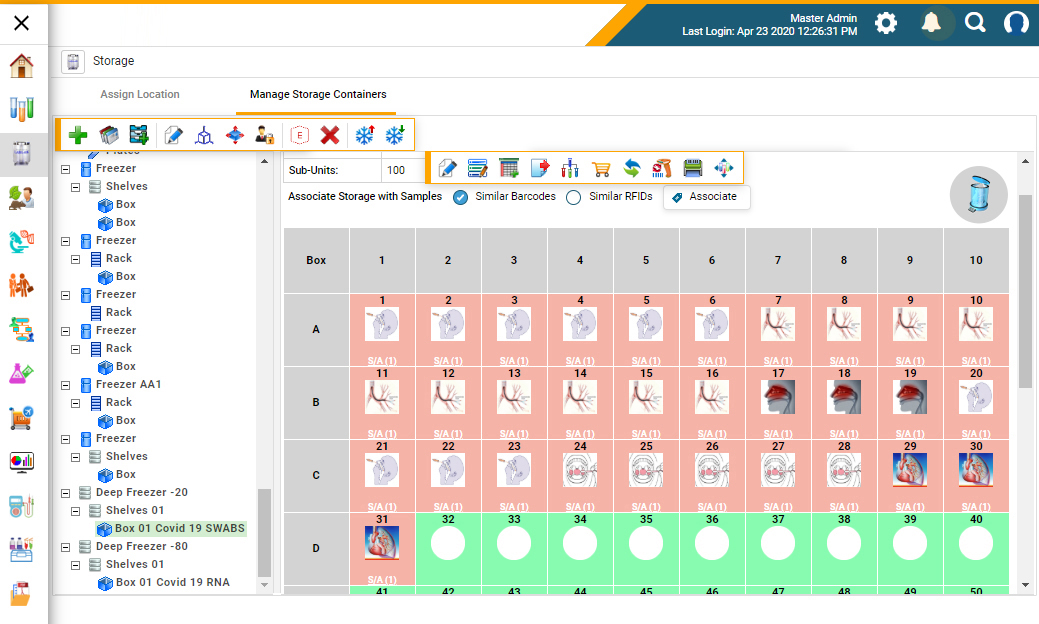
Figure 1: A biobank management system to mirror storage inventory of biobanks and to locate stored samples with ease (Figure courtesy of CloudLIMS)
3. Track Freeze-Thaw Cycles
Biological samples are particularly sensitive to changes in temperature, such as freeze-thaw cycles. Therefore, to ensure sample quality, it is essential that a biobanking LIMS tracks sample storage conditions, including freeze-thaw cycles, and alerts staff of any temperature deviations.
4. Comply With Regulatory Guidelines
Biobanks operate in a strict regulatory environment. A biobanking LIMS must have all the necessary features to comply with regulatory guidelines.
It must be able to track the location and chain of custody of all samples, with user identification, electronic signatures and time and date stamps to provide a full audit trail. It should also provide a mechanism to actively manage patient/donor consent and anonymize data and samples. Under U.S. and European guidelines, patients and donors must give voluntary, informed consent before biobanks can use their samples or data (5,6). Donors must also be able to withdraw that consent anytime.
To ensure compliance with biobanking regulatory guidelines, such as ISO 20387:2018, HIPAA, and ISBER Best Practices, a LIMS can also help biobanks manage documents related to validation of processes, SOPs and manage staff training and competency records. Furthermore, an effective biobanking LIMS can help schedule instrument calibration and manage maintenance data, ensuring the upkeep of instruments which is crucial for high-quality test results.
5. Manage Data and Ensure Data Security
Data management is another essential part of regulatory compliance. First and foremost, a LIMS must keep all patient/donor data secure with data encryption and controlled data access. Only authorized users may access this information. To provide a full audit trail, a biobanking LIMS must also allow biobanks to track all revisions and changes to documents and data, with user identification and time and date stamps.
6. Be User-Friendly, Update in Real-Time and Allow Scalability
A biobanking LIMS should have a user-friendly interface, be accessible but secure, and easy to search. For example, a cloud-based LIMS allows authorized users to access data, track samples and share information with collaborators, in real-time, from any device. When combined with regulatory-compliant data security, this provides biobanks with a flexible way to manage their inventory and data.
Lastly, a LIMS should be scalable – allowing biobanks to add more samples, more clients, more users and more sites as necessary.

Figure 2: A schematic representation of the essential features of a biobanking software solution (Figure courtesy of CloudLIMS)
Conclusion
Biobanks have the challenging job of managing large numbers of sensitive biological samples and vast amounts of protected health data within a strict regulatory environment. A biobanking LIMS allows biobanks to maintain sample quality by monitoring sample conditions in real-time throughout the sample lifecycle. A LIMS also ensures regulatory compliance by keeping data secure, limiting access to authorized users, providing a complete audit trail, and managing biobanking documentation and staff training. To select the right biobank information management system, biobank managers must consider the features described in the checklist above.
References
1. Health Insurance Portability and Accountability Act of 1996. U.S. Department of Health & Human Services. https://aspe.hhs.gov/report/health-insurance-portability-and-accountability-act-1996. Published August 21, 1996. Accessed December 22, 2020.
2. Paul, S. Empowering Biobanks to Comply with the EU GDPR for Personal Data Protection using a Cloud-based LIMS. CloudLIMS. https://cloudlims.com/lims-posters/empowering-biobanks-to-comply-with-the-eu-gdpr-for-personal-data-protection-using-a-cloud-based-lims.html. Published October 14, 2019. Accessed December 22, 2020.
3. ISO 20387:2018 Biotechnology – Biobanking – General Requirements for Biobanking. International Organization for Standardization. https://www.iso.org/standard/67888.html. Published August 2018. Accessed December 22, 2020.
4. Campbell LD, Astrin JJ, DeSouza Y, et al. The 2018 Revision of the ISBER Best Practices: Summary of Changes and the Editorial Team's Development Process. Biopreservation and Biobanking. 2018; 16(1): 3-6. doi: https://doi.org/10.1089/bio.2018.0001
5. Recommendation CM/Rec(2016)6 of the Committee of Ministers to member States on research on biological materials of human origin. Council of Europe. https://search.coe.int/cm/Pages/result_details.aspx?ObjectId=090000168064e8ff. Published 2016. Accessed December 29, 2020.
6. Electronic Code of Federal Regulations. US Government Publishing Office (GPO) https://www.ecfr.gov/cgi-bin/text-idx?m=12&d=18&y=2020&cd=20201223&submit=GO&SID=83cd09e1c0f5c6937cd9d7513160fc3f&node=pt45.1.46&pd=20180719#se45.1.46_1116. Published December 18, 2020. Accessed 29, 2020.
Shonali Paul is the chief operating officer at CloudLIMS.com

