Stressed-out Cells Are Risk to "Mini-brains"’ Modeling Potential
Complete the form below to unlock access to ALL audio articles.
Far from being the “brains-in-a-dish” touted by some sections of the media, cerebral organoids, hunks of neural tissue designed to act as in vitro models of neural processes, might actually fail to replicate even basic tenets of neuronal development. That’s the conclusion of a new study authored by researchers at UC San Francisco that appears in Nature January 29th.
“We find that organoids do not develop the distinctive cell subtypes or regional circuit organization that characterize normal human brain circuits. Since most human brain diseases are highly specific to particular cell types and circuits in the brain, this presents a grave challenge to efforts to use organoids to accurately model these complex conditions,” said Arnoid Kriegstein, a professor of neurology in the UCSF Weill Institute for Neurosciences, in a press release.
Kriegstein had raised similar queries about the cellular makeup of organoids back in October at the Society for Neuroscience conference in Chicago. There, he told a press conference, “The cell types are broadly similar to the ones you find in normally developing tissue, but the problem is that our genetic analysis is showing that they lack specificity, as though their identify is a bit confused.” This new research has shown the results of that confusion.
Jumbled-up organoids are cause for concern
The study arose following efforts by a Kriegstein lab postdoc, Dr Aparna Bhaduri, to fully record the gene expression programs that control brain development. Bhaduri’s project aims to create a resource that can be used to pin down what malfunctions in these programs underlie neurodevelopmental conditions like autism.
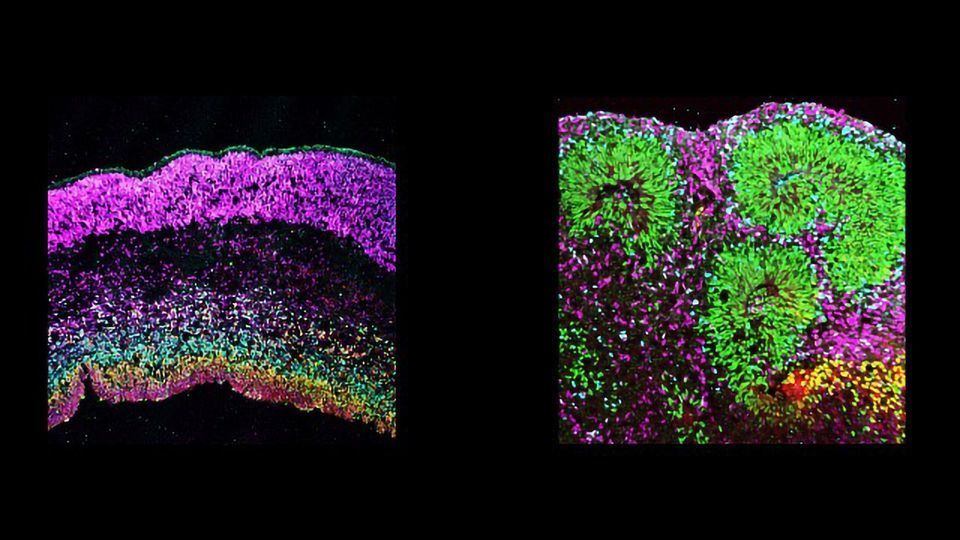
The organoids’ issues were highlighted when a fellow postdoc, Dr Madeline Andrews, compared Bhaduri’s gene expression maps to the levels found in the lab’s organoid store. In the not-quite-mini-brains, these carefully designed gene sequences, which are essential for correct brain development, were jumbled around.
This muddled-up genetic foundation had numerous outcomes in the organoids’ cells. Whilst broad cellular classes were present, the carefully organized subtypes and developmental structure that were seen in normal brain tissue were absent from the organoid cells.
“We were able to identify the major broad categories of cell types, but the normal diversity of subtypes – which play key role in the proper function of neural circuits – was lacking,” Kriegstein said.
235,000-cell study puts organoids in the spotlight
These results came from some mind-bogglingly extensive analysis: Kriegstein’s team analyzed 37 different organoids, extracting 235,000 cells in total. These organoids were generated using several different protocols and the lab even looked at data produced using eight other organoid protocols found in the wider literature. The messy organoid cells were present in all the organoid data analyzed.
In one experiment, the authors noted that even though organoid cortical neurons were able to maintain the specific identity of the area of the brain they would usually originate from, the spatial positioning of the cells went haywire, leaving different cell types mixed together throughout the organoid.
So why do organoid cells develop so strangely? Kriegstein’s team noted that levels of cellular stress were extremely high in organoid cells. These genes are usually activated in response to unpleasant environmental conditions.
How to bring calm to stressed-out organoids?
Kriegstein and his team used an innovative, if grisly, method to relieve the cells’ stress. If organoid cells were transplanted into the brains of mice, the cellular stress and odd developmental problems abated, and the cells started to resemble happy and healthy neural tissue. In turn, normal neural incubated in dishes with organoid tissue started to mimic the organoids’ developmental problems. Of course, given that one potential benefit of organoids is to reduce the number of animals used in research, this finding doesn’t offer a full solution to the problem.
Andrews is clear that the answer will take a field-wide effort: “Different groups have optimized how they culture organoids in lots of different ways, so the fact that we see these issues across organoids from different laboratories suggests it's probably going to take a pretty big overhaul to improve how organoids turn out.”
These changes will be essential if organoids are to reach their full potential as models of the brain, said Bhaduri, “Before we can use organoids to study these diseases and search for potential cures, we need to ensure they are actually modeling the brain circuits that are affected.”
But even jumbled up organoids can prove useful in research, so these findings should be seen as a setback rather than a reason to abandon ship altogether. What Kriegstein hopes is that this evidence should put paid to the idea that organoids could become sentient any time soon, “Some people have branded organoids as 'brains-in-a-dish' but our data suggest this is a huge exaggeration at this point,” said Kriegstein.


