Disruption of Normal Circadian Function in a Mouse Model of Tauopathy
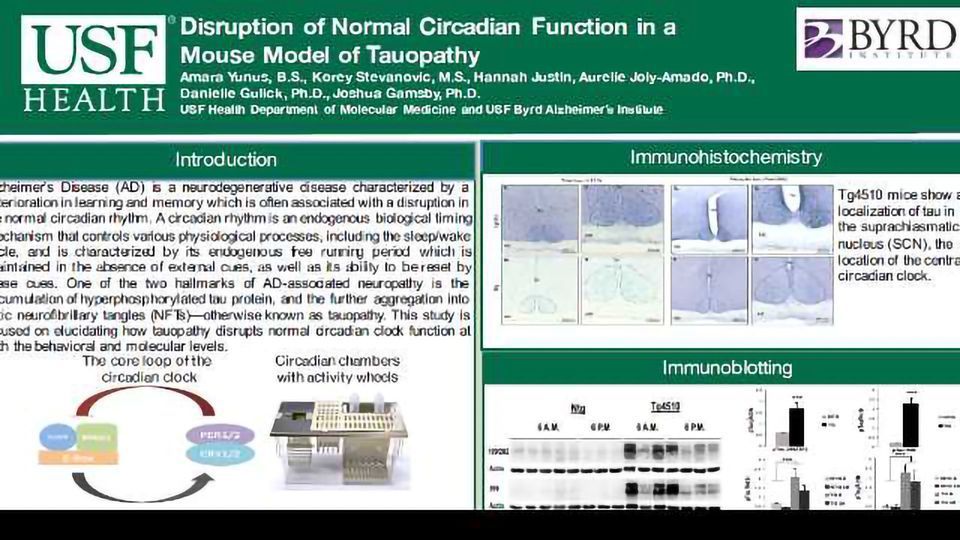
Objectives: Alzheimer’s Disease (AD) is a neurodegenerative disease characterized by a deterioration in learning and memory which is often associated with a disruption in the normal circadian rhythm. A circadian rhythm is an endogenous biological timing mechanism that controls various physiological processes, including the sleep/wake cycle, which is often disrupted in patients suffering with AD. One of the two hallmarks of AD-associated neuropathy is the accumulation of hyperphosphorylated tau protein, and the further aggregation into toxic neurofibrillary tangles (NFTs)--otherwise known as tauopathy. This study is focused on elucidating how tauopathy disrupts normal circadian clock function at both the behavioral and molecular levels.
Methods: Tg4510 mice are a double transgenic TET-off model of tauopathy in which the human mutant P301L allele tau (MAPT) is expressed in regions of the brain involved in learning and memory. The Tg4510 mice were housed in circadian chambers that measured activity activity on running wheels. Mice were entrained to an 12-hour light/dark (LD) cycle for 12 days followed by 12 days of a 24-hour dark/dark (DD) cycle. Mice were re-entrained to LD and then reintroduced to DD prior to sacrifice in order to collect brain tissue when the circadian clock is controlling sleep/wake behavior independent of external cues, such as light. Brain tissue was collected from the hypothalamus, the site of the master clock, and the hippocampus, a key site of learning and memory, at two time points: 6 a.m. and 6 p.m. Western blotting was used to analyze the expression of clock proteins (PER2, BMAL1), phosphorylated tau protein, and total levels of tau.
Results: We found both behavioral and molecular differences between our transgenic and non-transgenic mice. Tauopathy induces a longer circadian cycle in DD in Tg4510 mice at both 3 and 8 months of age, indicating an alteration in their circadian clock occurs during the early stages of tauopathy. We also found a disruption of the molecular clock proteins in the hypothalamus and hippocampus of Tg4510 mice.
Conclusion: We propose that the accumulation of hyperphosphorylated tau seen in AD may be associated with sleep and circadian disruptions, which can be seen at both behavioral and molecular levels.




