CD8+ T Cells

Complete the form below to unlock access to ALL audio articles.
Contents
T-cell lymphocytes play a critical role in cell-mediated processes that underlie adaptive immunity. When T-cell receptors (TCRs) found on naïve T cells recognize their specific antigen bound to major histocompatibility complex (MHC) class I proteins, they mature and proliferate into a specialized subset of T cell types that perform key immune functions. In this article, we focus on CD8+ T cells by characterizing their immunological phenotypes and the processes behind their cell-mediated immune functions.
What are CD8+ T cells?
Often called cytotoxic T lymphocytes (CTLs), CD8+ T cells belong to a subpopulation that expresses CD8 on their surface. CD8 is a dimeric co-receptor that enables CD8+ T cells to recognize peptides presented by MHC class I proteins.The major histocompatibility complex (MHC) is a region of DNA that contains a number of genes coding for glycoprotein molecules essential to immune system recognition of foreign material.
There are two major classes of MHC molecules. There are key differences between them.
In the thymus, successful binding between a naïve CD8+ T cell and an antigen-presenting cell (APC) stimulates the immature T cell to become an activated CD8+ T cell with cytotoxic functionality. With cytotoxic capabilities like the killing of intracellular pathogens and tumor cells, CTLs belong to a group of specialized lymphocytes involved in cell-mediated adaptive immunity.1 While CTLs are essential for a functional immune system, the dysregulation of CTLs can lead to the pathogenesis of organ-specific autoimmune diseases2 like diabetes or arthritis.
Where do CD8+ T cells originate?
CD8+ T cell development
Positive selection begins when TCRs bind to antigens in complex with MHC class I proteins presented by thymic cells. If this binding event is successful for CD8, the newly activated CD8+ T cells will continue differentiation into mature CTLs. Immature T cells unable to adequately bind to the antigen-MHC class I complex at this stage will undergo apoptosis. Negative selection occurs when immature T cells bind too strongly to self-antigens. Negatively selected T cells are programmed to undergo apoptosis, which eliminates their ability to become autoreactive against healthy cells.
CD8+ T cell activation
What do cytotoxic T cells do?
Once CD8+ T cells are activated, they undergo a cascade of replication and differentiation steps that primes them for a targeted immune response. CTLs that come into physical contact with targeted infected or malignant cells induce apoptosis, a form of programmed cell death carried out by cytotoxic enzymes and molecules.
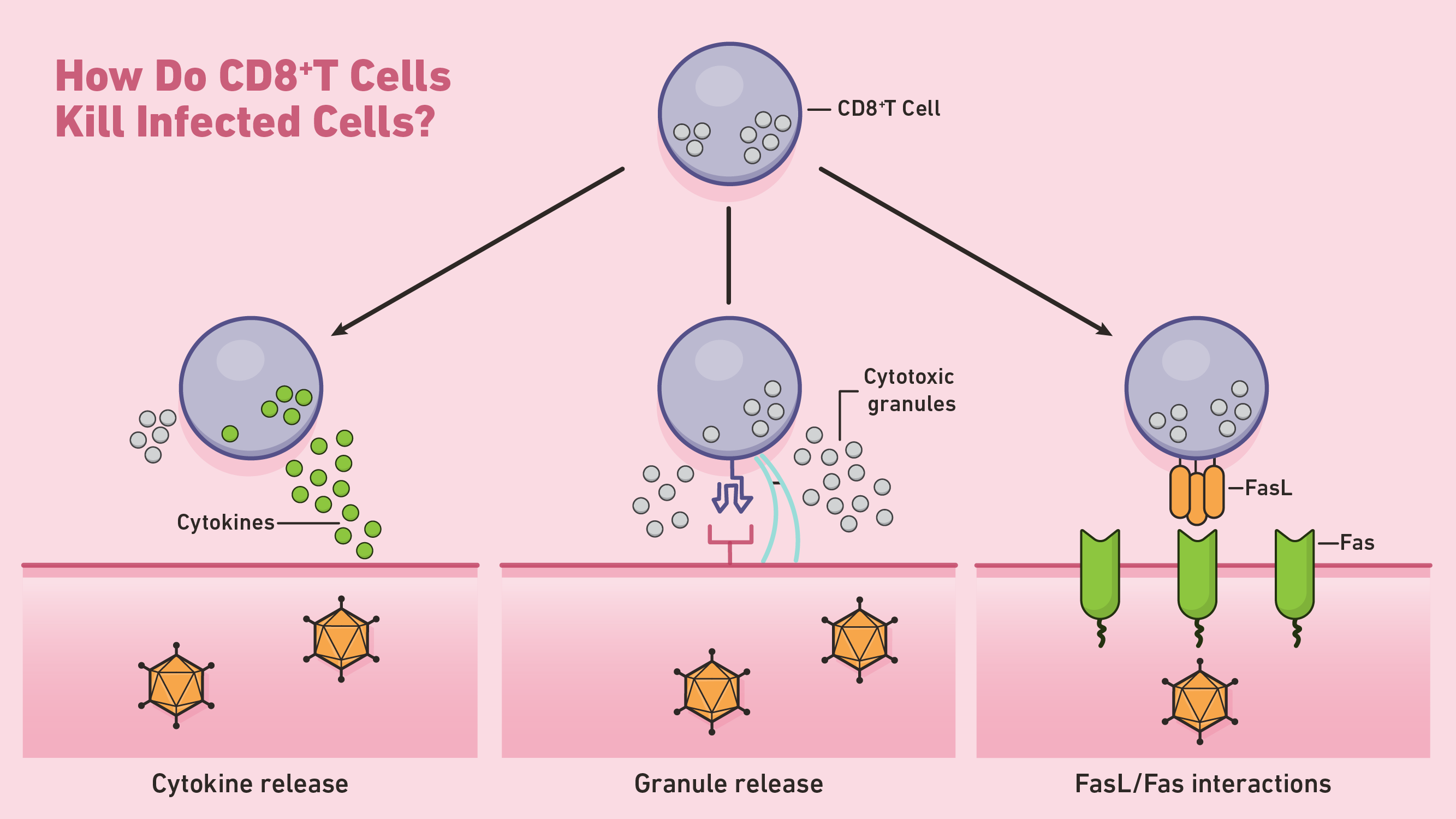
Figure 1: How do CD8+ T cells kill infected cells? Credit: Technology Networks
One way in which CD8+ T cells initiate apoptosis is through the secretion of perforin and granzymes, two types of cytotoxic proteins. Perforins are cytolytic proteins that form pores in the cell membrane of targeted cells. CTLs use these pores to direct the release of granzymes, a class of serine proteases that continue the process of apoptosis within the cytosol. These proteases shut down target cells by cleaving viral and cellular proteins involved in normal cell maintenance. Targeted cells undergoing apoptosis are then cleared away by nearby phagocytes.
CTLs can also induce apoptosis through Fas-Fas ligand interactions. Whenever Fas ligand (FasL) binds to the Fas receptor on targeted cells, a series of signaling molecules initiate apoptosis through the activation of caspase proteases. These caspases carry out the bulk of proteolysis throughout the latter stages of apoptosis.
In addition to directed apoptosis, CD8+ T cells can kill target cells indirectly through the release of cytokine factors like TNF-α. For example, type 1 CD8+ T cells (Tc1) can release IFN-γ, a cytokine capable of inhibiting viral replication and enhancing the presentation of specific antigens.4 At the end of their primary response, activated CD8+ T cells typically die by apoptosis.
CD8+ T cell subtypes

Figure 2: CD8+ T cell subtypes include Tc1, Tc2, Tc9, Tc17 and Tc22. Credit: Technology Networks
Memory CD8+ T cells are generally identified after a contraction of the effector cell population occurs. Certain effector cells remain after this contraction and become memory cells. The memory subset of CD8+ T cells can be categorized into three distinct groups: central memory cells, effector memory cells and tissue-resident memory cells. They are thought to be largely derived from the Tc1 subset1. These subsets are characterized by differences in their function, capacity to proliferate and location. Central memory cells (Tcm) are lymphoid-residing lymphocytes that typically respond to secondary infections through rapid proliferation. In humans, Tcm cells are phenotypically characterized by their co-expression of surface markers CD127, CD27 and CD28. Effector memory cells (Tem) circulate throughout the body ready to seek and kill antigen-matching target cells. Unlike their Tcm counterparts, these effector memory cells do not exhibit a robust ability to proliferate or self-renew in response to an infection. Lastly, tissue-resident memory cells (Trm) are mature CTLs that “stand by” for secondary infections at the original site of injury or invasion.
CD8+ vs CD4+ T cells
| Comparison | CD8+ T cells | CD4+ T cells |
| Type | Cytotoxic or “killer” cells | “Helper” cells |
| Key function(s) | Destroy infected or malignant cells | Mediate immune cell activity |
| TCR-MHC binding complex | CD8 + MHC class I | CD4 + MHC class II |
| Number of subtypes | Five | Six |
Where do CD8+ T cells fit into the broader immune response?
CD8+ T-cell regulation depends on a collection of stimulatory and inhibitory mechanisms mediated by TCRs. When CD8+ T cells are presented with their specific antigen, they undergo numerous clonal expansions that produce differentiated cell types required for an adaptive immune response. Independent of antigen presentation through TCRs, CD8+ T cells can indirectly attack pathogens or tumorous cells through the secretion of cytokines.6 However, unregulated CTL activity can contribute to autoimmune responses against healthy cells. A greater understanding of CD8+ T-cell processes in such cases could enhance our abilities to design effective vaccines or to manipulate cell-mediated immune responses in real-time.
References
1. Zhang, N, Bevan, MJ. CD8+ T cells: Foot soldiers of the immune system. Immunity. 2011;35(2):161-168. doi: 10.1016/j.immuni.2011.07.010
2. Deng Q, Luo Y, Chang C, Wu H, Ding Y. and Xiao R. The emerging epigenetic role of CD8+T cells in autoimmune diseases: A systematic review. Front. Immunol. 2019;10:856. doi: 10.3389/fimmu.2019.00856
3. Hoyer S, Prommersberger S, Pfeiffer IA, Schuler-Thurner B, Schuler G, Dörrie J, Schaft N. Concurrent interaction of DCs with CD4(+) and CD8(+) T cells improves secondary CTL expansion: It takes three to tango. Eur. J. Immunol. 2014;44(12):3543–59. doi: 10.1002/eji.201444477
4. Tau G, Rothman P. Biologic functions of the IFN-γ receptors. Allergy. 1999;54(12):1233–1251. doi: 10.1034/j.1398-9995.1999.00099.x
5. Zhu J, Paul WE. (2008). CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–69. doi: 10.1182/blood-2008-05-078154
6. Berg RE, Forman J. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr Opin Immunol. 2006;18(3):338-343. doi: 10.1016/j.coi.2006.03.010





