The Rationale Behind Social Distancing and Hand Washing

Complete the form below to unlock access to ALL audio articles.
In the time of COVID-19, the Internet is aflush with expert advice about social distancing.
At the same time, raw emotion has driven many to massive trips to the grocery store.
In addition to toilet paper, alcohol-based hand sanitizer is the favorite on everybody’s panic-shopping list. The sale of hand sanitizers has gone up almost 500%, in contrast to soap, which has gone up a paltry 70%.
So why do experts advise social distancing, and are hand sanitizers the answer to all our problems?
Why we should practice social distancing
Some viruses, such as Ebola and HIV, travel mainly via close, even intimate contact. Other viruses, such as coronaviruses, travel in droplets expelled from the original host via sneezing, coughing, or just speaking.
Unlike bacteria, viruses do not possess any metabolic machinery themselves. In other words, viruses can only survive for a limited time outside of the host and need to get to the next host quickly. If a potential host is standing within the range of travel by the virus-containing droplets, then they have a high probability of catching the droplets and becoming infected.
What is the range that the droplets can travel then?
In a study, researchers sampled the air in a hospital room at different distances from flu patients and found that health care providers could be exposed to infections even six feet from the patients. The finding refutes the previous belief that aerosol droplets can travel from 3 to 6 feet.
Another study even found that, instead of dropping directly from the mouth to the ground, the droplet continues to travel after its liquid component dries. As a result, virus particles can travel as far as 10 meters (30 feet).
One can deduce from these studies that a distance of 30 feet is safest. However, since maintaining such distance is not always practical, we can start with six feet when we practice social distancing.
The mathematical explanation of social distancing
How does social distancing reduce the spread of a virus?
The number of infections at any time (t) can be calculated as such:
N(t) = N0 e^(a*t)
N(t): the number of people infected at time t
N0: the starting number of infected people
a: infection (transmission) rate; a smaller a will lead to a smaller increase in the case number.
According to Professor Lou Bloomfield of the University of Virginia, one infected patient usually infects two to three others, or when a = 2 or a = 3.
When one patient infects two others, a = 2:
N(t) = N0 e^(2t)
When one patient infects three, a = 3:
N(t) = N0 e^(3t)
Like any other y = e ^ a (a > 1) exponential graph, the value of y will double quickly. In the case of virus infection, the number of cases will double in mere days, and the shape of the graph will look like the “exponential” part of the curve below.
Social distancing will reduce the number of persons a patient will infect to one (a = 1), so:
N(t) = N0 e^(t) = N0 e
resulting a flat line that looks like the “flattened” part of the graph below, where the spread of infection has slowed down significantly.
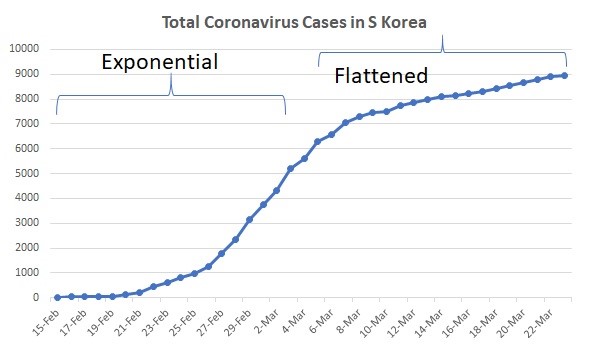
Figure 1: The total number of coronavirus cases in South Korea over time.
Data Source: https://www.worldometers.info/coronavirus/country/south-korea/.
Credit: Sylvia He.
Why is soap and water better than hand sanitizers?
The effectiveness of a sanitizing routine varies with different types of viruses.
There are two types of viruses: naked and enveloped. Naked viruses consist of genetic materials in a shell that is made up of protein building blocks (the capsid), and enveloped viruses have an additional coat (envelope) of fat molecules (lipid bilayer membrane) outside of the capsid.
The coronavirus, which causes COVID-19, is an enveloped virus. Once the viral envelope is damaged or destroyed, the virus will become inactive.
Soap, a detergent made up of fat molecules, can inactivate the COVID-19-causing virus easily by dissolving its lipid layer. A meta-study has shown water and soap to be more effective than waterless products for removing dirt and pathogens.
What about all the hand sanitizers that people are buying?
Although there is a study showing that highly concentrated alcohol (95%) is more effective than soap and water in deactivating naked virus, the point is moot here, since coronavirus is not a naked virus.
In addition, at 70% alcohol, the effectiveness of hand sanitizer will be less than that of highly concentrated and almost pure alcohol. A study has shown that while alcohol-based products achieve rapid and effective inactivation of bacteria, they are less effective against viruses.
Also, the usefulness of hand sanitizer is hampered by how it is used. People love sanitizers because they think they only need to use a tiny dollop each time. In the study mentioned above, a quantity of alcohol that was large enough for soaking was used for disinfection. If hand sanitizers are to achieve an optimal level of disinfection, one bottle, perhaps several, will be needed each time. Lastly, because soapy water is less viscous and does not evaporate quickly, they can achieve complete coverage of disinfection. In contrast, with hand sanitizers, which are more viscous and evaporate quickly, it is harder to ensure coverage.
The effectiveness and low-cost of soap and water are the reason the Center for Disease Control recommends soap and water over hand sanitizers, especially in scenarios involving known infections or infected patients. Sanitizers are an advised option when soap and water are unavailable or when the situation is potentially infectious.
Why we should cough into our elbows and not hands
Lastly, the reason that we are advised to cough into our elbows and not hands is not only that coughing into our hands is disgusting – it is –but that it is more effective in inactivating viruses.
While temperature, sunlight, changes in pH, and salinity affect the integrity of the viral envelope, moisture is also crucial.
A study has shown that viruses can survive on non-porous surfaces like light switches and tabletops longer than on porous surfaces like fabric and tissues because porous surfaces absorb moisture from the viruses and cause them to disintegrate.
Interestingly, the same study also finds the human skin, with its pH and porous nature, a poor shelter for the viruses. Still, we should wash our hands immediately, since we use them to touch other surfaces and people often. Regardless of how quickly our hands may disinfect themselves, it is still not worth the risk to us or others.



