Vaccine 101: What Are They, How Do They Work and What's the Latest Research?

Complete the form below to unlock access to ALL audio articles.
Vaccines: Simple definitions
A vaccine is a biological product that generates acquired immunity to certain infectious diseases.
Vaccines produce this protective mechanism by effectively training the immune system to both recognize and fight pathogens.
For the immune system to achieve this, vaccines are made from either dead, weakened or subunit forms of an organism that contain antigens which should not be able to cause disease.
The introduction of the vaccine triggers an immune response, whereby the body detects the antigens in the vaccine and B lymphocytes, a certain type of immune cell, respond as though the actual organism is trying to invade the body. The B lymphocytes multiply and eventually develop into either plasma cells or memory B cells.
The plasma cells produce antibodies that attach directly to and inactivate the organism to which you are being vaccinated against (a process known as the primary response). The body continues to produce antibodies and memory B cells for several weeks or longer post-vaccination, but eventually the antibody numbers decline, leaving the memory B cells that remain in the body long-term.
The premise of vaccination is that re-exposure to the infectious organism in the future will result in the memory B cells teaming up, rapidly multiplying and dividing into the antibody producing plasma cells. In the case of certain pathogens, a series of vaccinations are required in order to achieve a sufficiently strong immune response. Similarly, repeat vaccinations are required over time to create immunity against some infectious diseases.
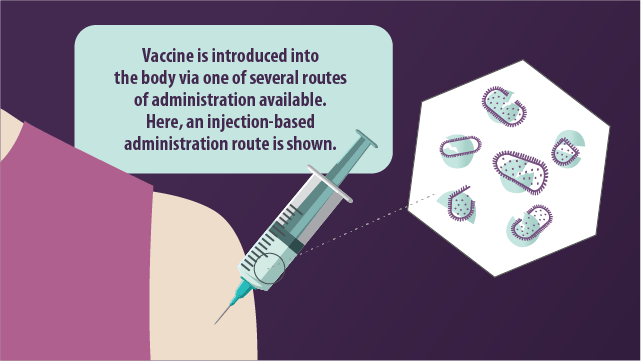


What types of vaccines exist?
- Live attenuated
Live yet weaker form of the pathogen, generates a strong cellular and humoral immune response. Rotavirus, chickenpox, and measles, mumps and rubella vaccines are examples of live attenuated vaccines.
- Inactivated vaccines
Inactivated pathogens that are therefore safe for inoculation. Hepatitis A, influenza and polio vaccines are examples of inactivated vaccines.
- Subunit, recombinant, polysaccharide and conjugate vaccines
Use a specific part of the pathogen, e.g. its protein, capsid or sugar to create a targeted immune response. These vaccines are used to protect against hepatitis B, human papillomavirus and shingles, to provide a few examples.
- DNA/RNA and viral vector
Parts of the pathogen’s genome are recombined into plasmids which are translated in host nuclei to make antigens.
In addition to antigens, vaccines also contain ingredients such as adjuvants, which enhance the immune response, preservatives and antibiotics which are necessary during the synthesis of subunit vaccines to select for correct strains or plasmid containing bugs, in addition to preventing contamination during the process of manufacturing.
Routes of vaccination
The path via which a vaccine is introduced to the body is known as the route of administration, of which there are several:
- Intramuscular injection
Whereby the vaccine is introduced into the muscle mass.
- Subcutaneous injection
Vaccine is injected into the area beneath the skin, above the muscle mass.
- Intradermal injection
Vaccine is injected into layers of the skin.
- Oral
A needle-free approach, the vaccine is administered via the mouth.
- Intranasal spray
A second needle-free option, the vaccine is administered through the nasal mucosa.
The route of administration is a contributing factor to the efficacy of a vaccine and so is a critical consideration for vaccine manufacturers.
Human vaccine preclinical testing and approval
The clinical development of a vaccine for humans follows the same general pathways as for other drugs and biologics. In the US, when a sponsor wishes to begin a clinical trial with a vaccine product, they must submit an Investigational New Drug (IND) application to the Food and Drug Administration (FDA).
The IND must provide the following information:
- A description of the vaccine
- Methods of manufacturing the vaccine
- Data from quality control (QC) tests for release
- Information regarding the safety of the vaccine
- Data that demonstrates the vaccine can elicit immunogenicity in animal testing
- Proposed protocol for human clinical trials
As with any drug or biologic, a vaccine must undergo clinical trials, and these are often undertaken in three phases.
Vaccine licensing
If the vaccine successfully passes through the three phases of clinical trials, a Biologics License Application (BLA) can be submitted to The Center for Biologics Evaluation and Research. After submitting, the BLA, the sponsor and the FDA can also choose to present their information to the FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC).
At this time point, a pre-approval visit will take place whereby the manufacturing site of the vaccine is assessed whilst production of the vaccine is in process. After licensing, the product is continually monitored by the FDA, who require samples of each vaccine lot for testing.
Many vaccines also undergo Phase IV studies post-marketing to assess any adverse effects once the vaccine has been administered to the general population. In addition, duration of immunity studies are undertaken to assess the requirement for repeat vaccinations.
Currently licensed vaccines
A wide variety of vaccines are now marketed and available for different infectious diseases across the globe.
The FDA maintain a current list of vaccines that are approved for use in the United States.
The UK immunization schedule lists the routine schedule for vaccination at different time points across an individual's life. Examples include the 6-in-1 vaccine given to babies at 8, 12 and 16 weeks, the influenza vaccine and shingles vaccine often administered in adulthood and the pertussis vaccine recommended for pregnant women.
Vaccine hesitancy
Vaccine hesitancy is defined by the World Health Organization as "the reluctance or refusal to vaccinate despite the availability of vaccines". It was listed as one of the top 10 threats to global health in 2019. The refusal to vaccinate leads to the rise of diseases that could otherwise be avoided such as measles, which has seen a 30% rise in global cases. Research shows that it's difficult to correct vaccine misinformation and suggests that communication by national public health authorities may be a key factor to challenge declining vaccination numbers.
Vaccine research, industry insights and future steps
Whilst vaccines have helped to combat many different infectious diseases over recent years, including the eradication of smallpox and rinderpest, there are still hurdles to overcome.
One example is in neonatal and early life immunization. The main challenge here is to identify vaccine formulations and immunization strategies capable of inducing a sufficient cell-mediated immune response. A second issue is age-associated changes in vaccine response.
An evolving area of focus in vaccine research has been on alternative vaccine delivery systems, such as liposomes, to improve antigen stability and immunogenicity.
Funding is also a crucial issue in vaccine development. The Coalition for Epidemic Preparedness Innovation (CEPI), a collaboration between government, philanthropy, industry and civil society, launched in 2017. The aim of CEPI is to both finance and coordinate the development of vaccines against infectious diseases, with the hope that such vaccines can be used in outbreaks before they are declared emergencies.








