Tailoring Flow Reactor Equipment to the Particular Chemistry at Hand

Complete the form below to unlock access to ALL audio articles.
André de Vries received a PhD in Organic Chemistry, under the supervision of Ben L. Feringa at the University of Groningen, specializing in asymmetric catalysis. He has over 20 years of experience in chemical process R&D, first as part of the DSM Innovative Synthesis Research Center, which is now known as the “spin out” company InnoSyn BV. He currently holds the position of commercial director at InnoSyn BV. André’s main responsibility is to convince potential customers to collaborate with InnoSyn for process development towards complex chemicals and advanced intermediates.
We recently spoke to André to learn more about the considerations to make when scaling up chemical processes. He outlines the advantages of flow chemistry over batch processes and summarizes the focus of his upcoming presentation on designing and manufacturing the best fit for purpose flow reactor for any type of chemistry.
Q: What advantages does flow chemistry offer over batch processes?
A: There are several advantages:
- Micro- and millimeter sized channels have a much higher surface-to-volume ratio than batch vessels, intensifying heat and mass transfer dramatically, and allowing highly exothermic reactions to be executed (almost) isothermally.
- Thanks to a continuous operation the volumes of flow reactors are typically several magnitudes smaller. This allows significantly lower hold-up of hazardous and unstable reagents, next to flexibility in production volume.
- The low volume of flow reactors enables safe and cost-efficient pressurization. Toxic gases, previously avoided on large scale, can be safely used as reagents. Moreover, due to the absence of headspace it is often possible to dissolve the gas in the continuous liquid phase, thus removing the gas-liquid transfer barrier. Finally, increasing the pressure allows superheating of common low-boiling solvents to speed up the reaction kinetics and simplify the downstream processing.
- Process monitoring and control is improved with continuous processing, leading to lower level of impurities.
Q: What considerations need to be made when converting a lab scale chemical process to an industrial scale?
A: Ideally the development and scale up of a process requires a multi-disciplinary approach from the very start, so with a group of experienced chemists and engineers. To identify possible bottlenecks as well as the-most-fit-for-purpose solution, one will need to know:
- The kinetics of the reaction
- The speed of mixing required
- The heat generated and the heat transfer required to maintain isothermal conditions throughout the reactor.
In other words, with sufficient data one can define or design the best fit for purpose reactor for any type of chemistry.
Q: You are speaking at the 2019 “Flow Chemistry and Continuous Processing Conference” this April, could you tell us about the focus of your talk?
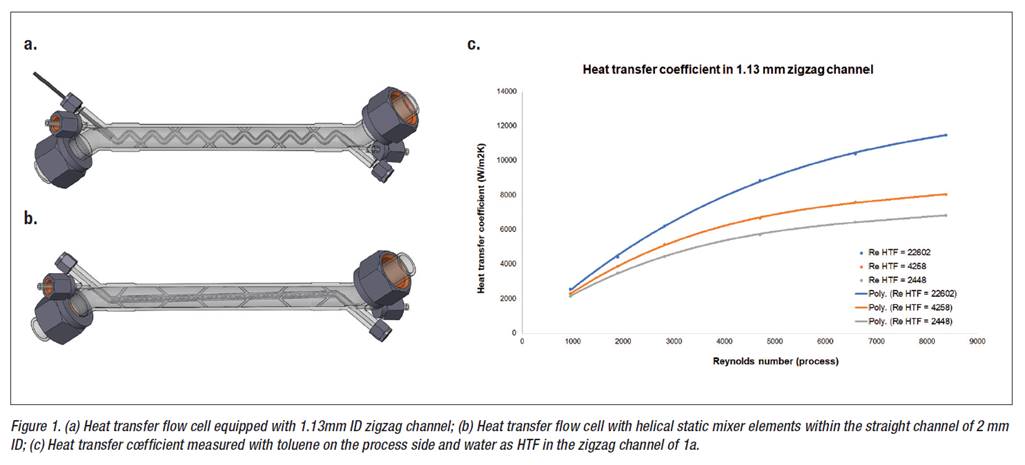
A: Rather than using a specific bought and installed flow chemistry equipment (available from several vendors), by adapting the conditions of reactions to fit into the particular capabilities of the flow reactor at hand, we at Innosyn prefer to tailor the specific flow reactor equipment to the particular chemistry at hand. With the accumulated data (see e.g. Figure 1 below) – kinetics of the reaction, the speed of mixing required, the heat generated, and the heat transfer required to maintain isothermal conditions – one can in principle design and manufacture the best fit for purpose flow reactor for any type of chemistry.
Additive manufacturing (3D metal printing) enables this goal since it provides almost an unlimited freedom of design and its quick iteration if needed. Several examples of the manufacturing of flow chemistry equipment will be highlighted, particularly focusing on improved heat transfer, and on static mixer devices. The use of those in organometallic chemistry (very fast, highly exothermic) and two sodium-azide chemistry examples (alkylhalide --> alkylazide substitution; assembling a substituted triazole) will be discussed.
Dr André De Vries was speaking to Laura Elizabeth Lansdowne, Science Writer for Technology Networks.




