Guardians of the Synapse
Complete the form below to unlock access to ALL audio articles.
Salk researchers have found, for the first time, that a blood-clotting protein can, unexpectedly, degrade nerves—and how nerve-supporting glial cells, including Schwann cells, provide protection. The findings, published March 14, 2019, in the journal PLOS Genetics, show that Schwann cells protect nerves by blocking this blood-clotting protein as well as other potentially destructive enzymes released by muscle cells. The work could have implications for diseases as diverse as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Alzheimer’s disease or schizophrenia.
“This is the first study to show that a molecule typically associated with blood clotting, thrombin, has a biological function outside of the liver system and plays an important role in nerve degeneration,” says Salk Professor Kuo-Fen Lee, senior author of the paper. “We further showed that Schwann cells can protect nerves against thrombin. The results were a complete surprise and raise intriguing questions about how synapses are formed and maintained in both health and disease.”
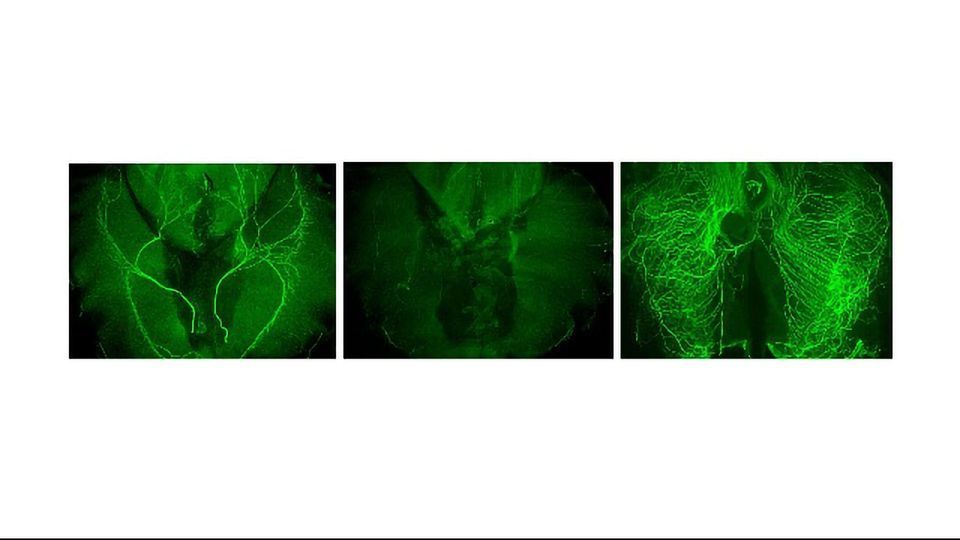
Schwann cells create a protective insulation around the threadlike projections of nerves called axons, and help form synapses, contacts where chemical signals are passed between cells. To better understand the role of Schwann cells in nerve health, the Salk team studied a specific synapse called a neuromuscular junction (NMJ), which interfaces between Schwann cells, nerves and muscles.
In the absence of Schwann cells, the NMJ synapse in a mouse model degenerated after two days, confirming their role in synapse growth. The researchers found that without Schwann cells, acetylcholine—the signaling molecule in the NMJ—was a major culprit in why the nerves degraded. When the researchers did a deeper dive to find out why, they uncovered a previously unknown mechanism: left to its own devices, acetylcholine prompts muscle cells to release a blood-clotting protein called thrombin, among other enzymes, that degrade the nerve. In healthy nerves, Schwann cells release molecules that block thrombin to protect their synapses.
“We were surprised that Schwann cells maintain developing neuromuscular synapses indirectly by inhibiting negative factors released from active muscle. One of these factors is thrombin, best known for its role in forming blood clots,” says former Salk researcher Thomas Gould, first author of the paper and now an assistant professor at the University of Nevada Reno School of Medicine.
To confirm the impact of thrombin on the NMJ, the researchers looked at a mouse model where thrombin was absent or nonfunctioning, and found that these mice experienced less nerve axon degeneration. These results affirm that thrombin plays a role in nerve axon degeneration.
“This study provides an understanding of the genetic and molecular pathways that alter synapse development and maintenance,” says Lee, holder of the Helen McLoraine Chair in Molecular Neurobiology. “The next step is to understand the mechanism of how thrombin and other enzymes destroy the synapse—with the eventual goal of creating an intervention for diseases—such as ALS, MS and Alzheimer’s—where thrombin accumulation or dysregulation has been implicated.”
This article has been republished from materials provided by the Salk Institute. Note: material may have been edited for length and content. For further information, please contact the cited source.
Reference: Gould, T. W., Dominguez, B., Winter, F. de, Yeo, G. W., Liu, P., Sundararaman, B., … Lee, K.-F. (2019). Glial cells maintain synapses by inhibiting an activity-dependent retrograde protease signal. PLOS Genetics, 15(3), e1007948. https://doi.org/10.1371/journal.pgen.1007948



